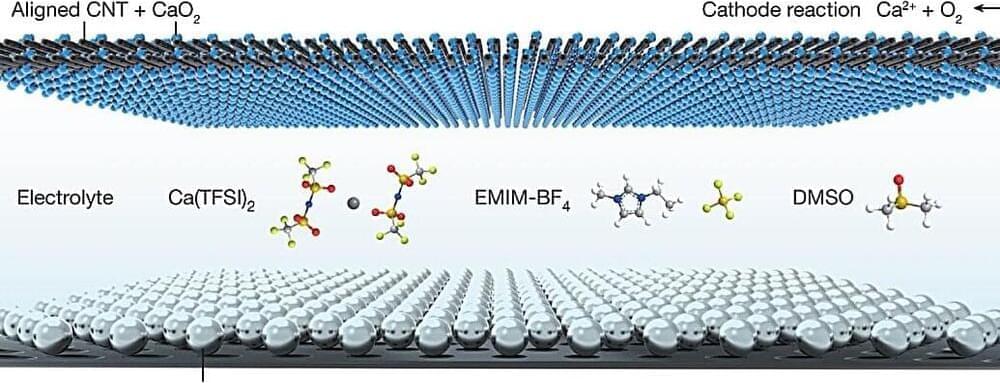
A multi-institutional team of Chinese engineers has developed a proof-of-concept calcium-based battery that withstands 700 charge cycles at room temperature. In their paper published in the journal Nature, the group describes the challenges they addressed in developing the battery and what they have learned about the possible use of calcium-based batteries in consumer products in the future.
The current standard for rechargeable batteries used in consumer products is lithium. But because it is a rare material and has issues such as poor aging and the need to prevent overcharge, scientists have been looking for a suitable replacement. One such material is calcium, which is 2,500 times as abundant as lithium.
Prior research has suggested rechargeable batteries based on calcium should be possible if problems can be resolved. One of the biggest challenges is finding suitable electrolyte and electrode materials that can provide stability and safety.