The three-dimensional (3D) organization of chromosomes is emerging as an important determinant of multiple cellular processes. We now show that 3D chromatin structures, maintained by the Polycomb complex, record epigenetic perturbation events.



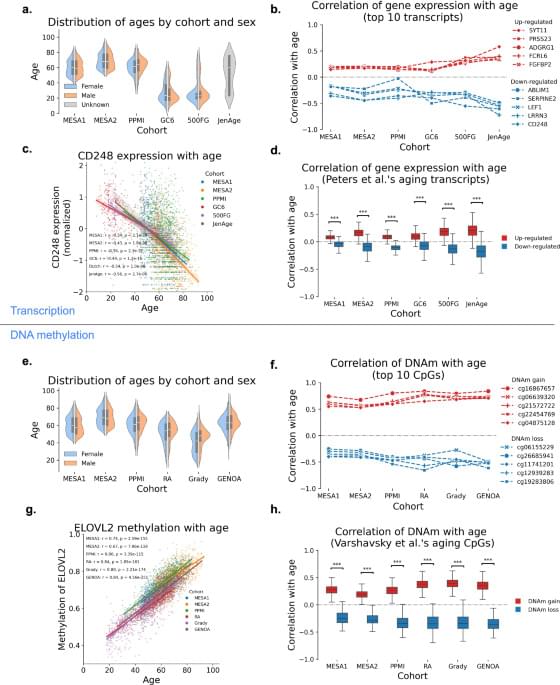
In a study published today, Friday, February 13, 2026, in the journal Nature Aging, researchers show that blood-based biomarkers can support accurate dementia diagnosis across diverse populations when integrated with cognitive and neuroimaging measures. Blood-based biomarkers are emerging as one of the most promising advances for the global diagnosis of dementia, including Alzheimer’s disease and frontotemporal lobar degeneration. These tests offer a more accessible, scalable, and cost-effective alternative to traditional diagnostic tools such as brain imaging or cerebrospinal fluid analysis.
However, most blood-based biomarkers have been developed and validated primarily in relatively homogeneous populations. Genetic background, overall physical health, and environmental and social exposures can substantially influence biomarker levels, raising concerns about how well these tests perform across diverse populations worldwide.

The new study, published in the journal Nature, provides new insights by demonstrating that while different mutations affect the developing brain in initially distinct ways, they increasingly impact overlapping molecular pathways as development progresses.
Researchers monitored the gene expression of the organoids over 100 days as they developed, which allowed researchers to observe how genetic changes affect brain during the critical early development windows.
Early in development, each genetic form showed distinct molecular signatures. However, as the organoids matured, these different mutations increasingly affected similar biological processes, particularly those involved in neuronal maturation and synapse formation.
The researchers identified a network of genes involved in regulating gene expression and chromatin remodeling, which is the process by which DNA is packaged and made accessible for reading. This network appears to play a central role in this convergence. Using CRISPR technology to individually reduce the activity of these regulatory genes in neural cells, the team confirmed that many of them control downstream pathways were previously linked to autism.
Notably, the study found few consistent molecular changes in organoids derived from individuals with idiopathic autism, likely reflecting the highly complex genetic architecture of autism that doesn’t involve major mutations. This finding underscores the need for much larger studies to understand the more common, polygenic forms of autism. ScienceMission sciencenewshighlights.
The researchers have created a comprehensive map showing how eight different genetic mutations associated with autism spectrum disorder affect early brain development, providing new insights into the ways diverse genetic causes may lead to shared features and symptoms of the disorder.

A positive newborn screening for spinal muscular atrophy (SMA) is currently considered a medical emergency. Without early treatment, severe disability or death in infancy are likely. However, research findings from Germany and Australia now show that in rare cases, a positive screening result can be a genetic false alarm. Researchers have discovered that functional tests in a zebrafish model may enable fast and reliable clinical decision-making in cases of unclear genetic findings.
The study “SMN1 variants identified by false positive SMA newborn screening tests: Therapeutic hurdles, and functional and epidemiological solutions” was published in the American Journal of Human Genetics and another study, “Clinical relevance of zebrafish for gene variants testing. Proof-of-principle with SMN1/SMA,” in EMBO Molecular Medicine. The collaborative research team was led by Professor Dr. Brunhilde Wirth, Director of the University of Cologne’s Institute of Human Genetics and Principal Investigator at the Center for Molecular Medicine Cologne (CMMC) and Dr. Jean Giacomotto from Griffith University’s Institute for Biomedicine and Glycomics, Brisbane, Australia.
The scientists examined two newborns—a girl from Germany and a boy from Australia—in whom routine screening initially failed to detect the SMN1 gene. A missing SMN1 gene is the main genetic trigger of SMA. This diagnosis would normally result in immediate treatment, as it would be assumed that the child’s life is in danger. However, further genetic analysis revealed a surprising finding: both children carried rare SMN1 variants that had not been detected by the screening test. It remains unclear whether these variants cause the disease.

What are the genetic origins of early-onset obesity?
Studying a cohort of young adults in China, researchers in Science TranslationalMedicine performed deep sequencing and identified a loss-of-function variant in the gene TUB that impairs sensitivity to leptin.
Rare human TUB variants impair leptin sensitivity through disruption of STAT3 activation, leading to hyperphagic obesity.

Neutrophils are known as first responders to threatening infections and feature prominently in the microenvironment of tumors to resist cancer progression. Though neutrophils have been linked to the growth of multiple cancers, such as lung and breast, these cells can assume multiple functional states.
In a new study published in Cancer Cell titled, “ CCL3 is produced by aged neutrophils across cancers and promotes tumor growth,” researchers from Ludwig Institute for Cancer Research in Lausanne have discovered a gene expression program executed by tumor-associated neutrophils (TANs) and a corresponding biomarker that uniformly support cancer cell survival and tumor progression across human and murine tumors.
Results demonstrate that TANs characterized by this conserved genetic program are a central variable of the tumor microenvironment (TME) linked to cancer progression. The authors also identify an associated marker, CCL3, as key to supporting cancer growth.

When a cell divides, it performs a feat of microscopic choreography—duplicating its DNA and depositing it into two new cells. The spindle is the machinery behind that process: It latches onto chromosomes (where DNA is stored) and separates them so they can settle into their new homes. This tricky process can sometimes go wrong, causing infertility, genetic disorders, or cancer.
Scientists have a good understanding of what spindles are made of: long, thin rods called microtubules as well as a variety of associated motor proteins. However, how these microtubules interact and organize to guide the spindles’ function has remained a mystery.
One approach to understand how the spindle self-organizes is to treat it like an active liquid crystal. Liquid crystals, like spindles, are made up of elongated subunits. Unlike liquid crystals in LCD displays, which require an external electric field to reorient their subunits, spindles are active materials that generate forces internally.

WASHINGTON (AP) — Scientists are testing an entirely new way to fight heart disease: a gene-editing treatment that might offer a one-time fix for high cholesterol.
It’s very early stage research, tried in only a few dozen people so far. But gene-editing approaches being developed by two companies show hints that switching off certain genes could dramatically lower artery-clogging cholesterol, raising hopes of one day being able to prevent heart attacks without having to take pills.
“People want a fix, not a bandage,” said Dr. Luke Laffin, a preventive cardiologist at the Cleveland Clinic. After co-authoring a promising study published in the New England Journal of Medicine, he said he was flooded with queries about how to participate in the next clinical trial.