Sep 5, 2022
Easing pain at the pump with food waste: New method for making biodiesel fuel
Posted by Shubham Ghosh Roy in categories: chemistry, climatology, sustainability
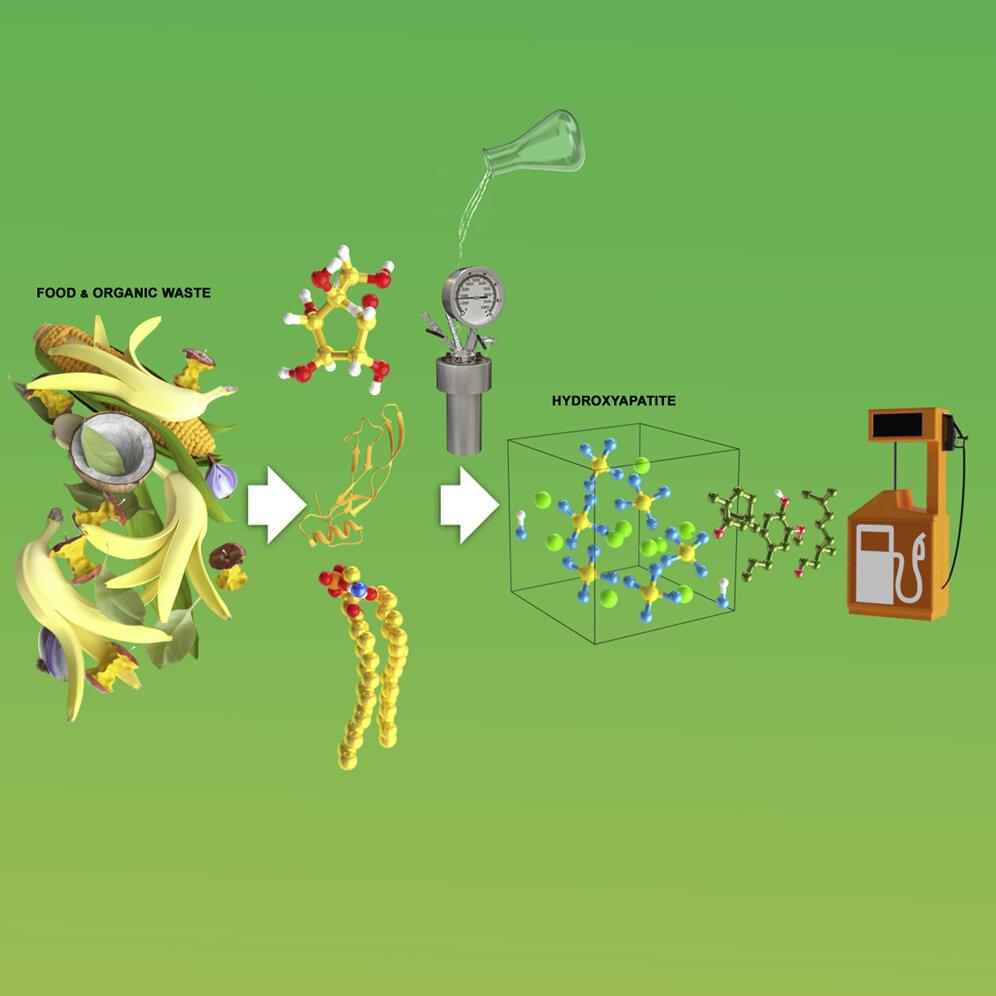
With gas prices soaring and food costs pinching family budgets, an interdisciplinary team of researchers at WPI is looking at ways to use food waste to make a renewable and more affordable fuel replacement for oil-based diesel. The work, led by Chemical Engineering Professor Michael Timko, is detailed in a new paper in the journal iScience.
“By creating a biodiesel through this method, we’ve shown that we can bring the price of gas down to $1.10 per gallon, and potentially even lower,” said Timko.
The Environmental Protection Agency estimates that, in 2018 in the United States, about 81% of household food—about 20 tons—ended up in landfills or combustion facilities. Food waste is also a major contributor to climate change: once it’s placed in landfills, it emits methane, a greenhouse gas.