Sep 18, 2023
A modern digital light processing technology to 3D print microfluidic chips
Posted by Saúl Morales Rodriguéz in categories: 3D printing, bioengineering, biotech/medical, chemistry, computing
Conventional manufacturing methods such as soft lithography and hot embossing processes can be used to bioengineer microfluidic chips, albeit with limitations, including difficulty in preparing multilayered structures, cost-and labor-consuming fabrication processes as well as low productivity.
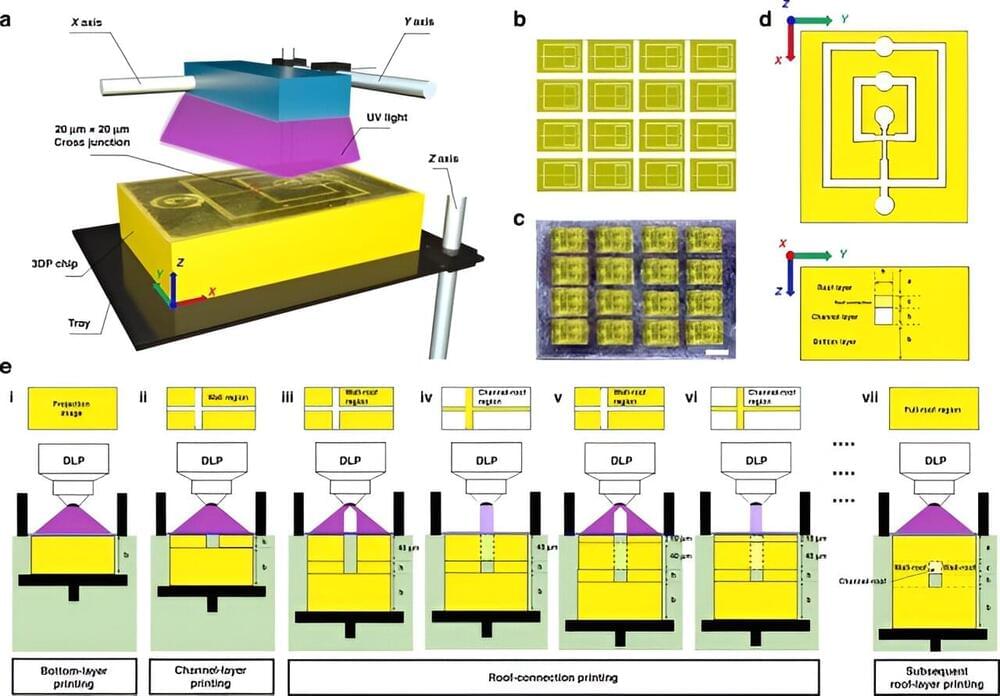
Materials scientists have introduced digital light processing as a cost-effective microfabrication approach to 3D print microfluidic chips, although the fabrication resolution of these microchannels are limited to a scale of sub-100 microns.
In a new report published in Microsystems and Nanoengineering, Zhuming Luo and a scientific team in biomedical engineering, and chemical engineering in China developed an innovative digital light processing method.