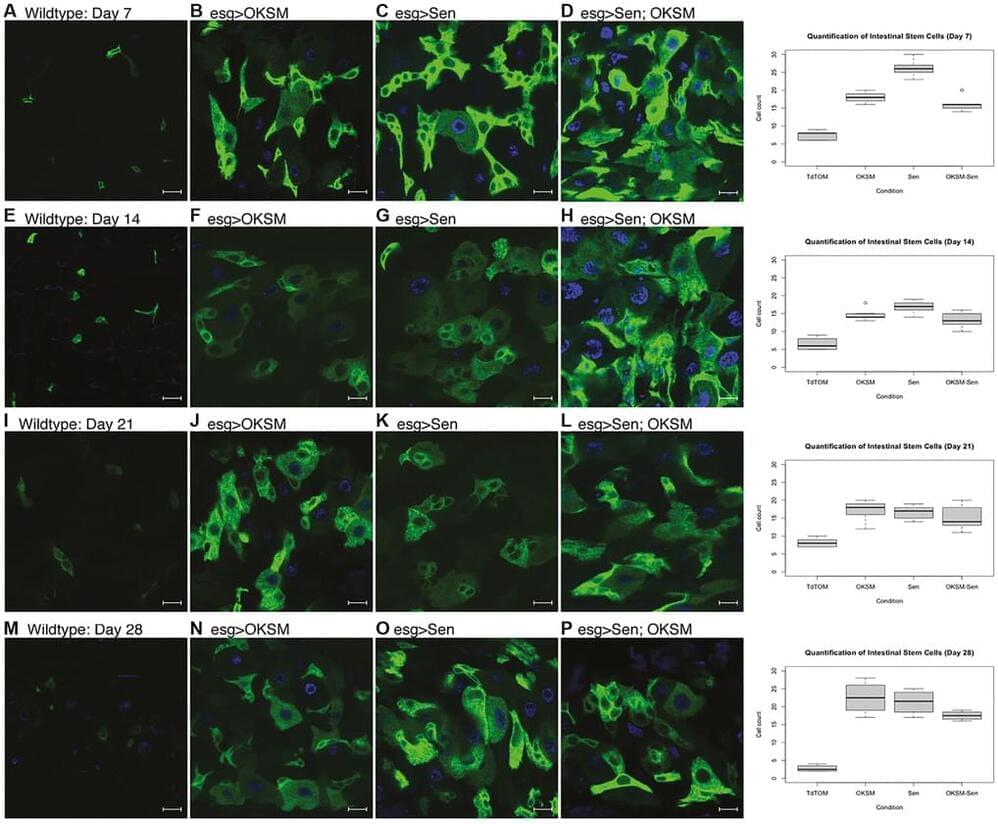
To test the interaction between senolytic removal of senescent cells and cellular reprograming, we designed a model combining these two interventions in an inducible overexpression system in Drosophila. First, we used the four Yamanaka factor based OKSM approach as this had been previously shown to induce pluripotent stem cells in mice [7], humans [29 – 31] and non-mammalian vertebrate and invertebrate species [32]. To make a senolytic factor for Drosophila, we took advantage of the mouse sequence (FOXO4-DRI [22]) to design an orthologous peptide based on the Drosophila foxo (forkhead box, sub-group O) gene [33]. We then characterized effects of these two interventions independently as well as in combination.
We began by looking at the effect of OKSM and Sen on stem cells in an intestinal stem cell (ISC) model [34, 35]. We chose to investigate phenotypic effects specifically in the digestive system of Drosophila (Supplementary Figure 1). As in mammals, the Drosophila gastric lining has a high turnover of cells which is enabled by stem cell pools that replenish the epithelia [34]. Age-dependent loss of stem cells and degradation of barrier function has been shown to contribute to age-dependent functional decline and mortality in Drosophila [36]. The Drosophila gut is composed of four cell types: enterocytes (ECs or absorptive cells), enteroendocrine (EEs or secretory cells), enteroblasts (EBs or transit amplifying cells) and intestinal stem cells (ISCs).








Comments are closed.