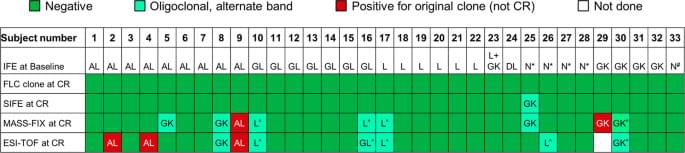
The Mayo Foundation Institutional Review Board (IRB) approved the study. All patients gave written informed consent to have their medical records reviewed and samples analyzed according to IRB requirements and federal regulations. Patients were eligible for this retrospective study if they: were diagnosed with AL amyloidosis between January 2000 and May 2015; were classified as amyloidosis complete hematologic response by immunofixation electrophoresis (IFE), serum free light chain (FLC) by consensus criteria;6,7 had a negative bone marrow by six-color flow cytometry; and had both a stored research sample prior to starting a line of therapy and a repeat sample while in complete hematologic response. The diagnosis of amyloidosis was made by Congo red with green birefringence under polarized light; the typing of the amyloid was with immunohistochemical stains or proteomics8,9. Supplementary Figure 1 is a consort diagram illustrating patient selection. Median time from institution of therapy to complete response (CR) sample was 18 months (interquartile range 9.1, 20 months).
The serum IFE (SIFE), urine IFE (UIFE), FLC, and bone marrow measurements were done as part of routine clinical practice as previously described4,5. Urine samples were concentrated to a maximum of 200× to achieve final concentrations of urine protein between 20 and 80 g/L4,5. The FLC assay (Freelite™, The Binding Site Ltd.) was performed on a Siemens BNII nephelometer10, and an abnormal FLC result was defined as an abnormal FLC κ/λ ratio. Bone marrow clonality was determined by six-color flow cytometry11. This method has sensitivity of ~10−4 to 10−5.
For MASS-FIX, immunoglobulins were enriched from serum using camelid-derived nanobodies directed against the heavy-chain constant domains of IgG, IgA, and IgM or the light-chain constant domains of κ and λ (Thermo Fisher Scientific)4,5. The +1 and +2 charge states of the light chains and heavy chains were measured by configuring the mass spectrometer to analyze ions between an m/z of 9000–32,000 Da.








Comments are closed.