Earth will be visited by several asteroids over the Fall Equinox, including one traveling at 27 times the speed of sound.




UK startup Fering is gearing up to build electric vehicles for cross-continental explorers. It’s starting out with the Pioneer, a go-anywhere brick outhouse of a thing designed for monster range figures under the most extreme circumstances on Earth.
Cybertrucks may be all well and good for your average camping trip, but they’re not designed for the kinds of extreme treatment the Pioneer wants to take on as a low-emissions alternative for explorers, adventurers and emergency services teams.
For starters, the lithium-ion batteries found in most EVs can’t handle extreme temperatures, so instead Fering has gone with a lithium-titanate-oxide (LTO) battery pack. These have advantages and drawbacks; they’re renowned for extremely long life cycles, they can charge quickly and they work from-40 to 60 °C (−40 °F to +160 °F), so they can handle just about anything shy of an Antarctic winter.
Four times faster than existing chemical rockets
In the seven months NASA estimates it would take to fly humans to Mars, any number of catastrophic failures could occur. That’s why Díaz said in a 2010 interview with Popular Science that “chemical rockets are not going to get us to Mars. It’s just too long a trip.” A conventional rocket must use its entire fuel supply in a single controlled explosion during launch before propelling itself towards Mars. There is no abort procedure, the ship will not be able to change course, and if any failure occurred, mission control would have a 10-minute communications delay, meaning they could find themselves helplessly watching on as the crew slowly dies.

Mars is the solar system’s near-miss world. Earth may have gotten everything right when it came to sustaining life—atmosphere, water, proximity to the sun. Mercury, Venus and the outer planets, with their extreme temperatures and inhospitable chemistry, may have gotten everything wrong. Mars, on the other hand, came so close, yet fell short.
Thanks to data from rovers and other spacecraft, we know that the Red Planet once fairly sloshed with water—with dry deltas, riverbeds, and sea basins stamped into its surface. But 4 billion years ago, the Martian core cooled, shutting down the dynamo that sustained its magnetic field. That left the planet vulnerable to the solar wind, which clawed away the atmosphere, and allowed the Martian water to sputter into space. Before long—in geological terms—the planet was a desert.
At least that’s long been the thinking. But a new paper published Sept. 20 in the Proceedings of the National Academy of Sciences suggests otherwise. According to the new research, Mars was doomed from the start. Its small size—about half the diameter of Earth and less than one-ninth the mass—simply never produced the gravitational muscle to allow the planet to hold onto either its air or its water. With or without a magnetic field, Mars was destined to die.

The firm is looking into how a micro-nuclear reactor could be used to propel rockets while in space at huge speeds and how that technology could then be redeployed to provide energy for drilling, processing, and storage for “Moon mining” and possibly “Mars mining.”
Dave Gordon, head of the company’s defense division, said this work is possible thanks to Jeff Bezos and Elon Musk and their respective space companies.
He added that’s Rolls-Royce is the only company on the planet that does mechanical, electrical, and nuclear and a full end-to-end lifecycle of nuclear capability. He also noted that the firm could use its experience in developing nuclear-powered submarines for the Royal Navy for 60 years to apply what it learned to spacecraft since submarines and spacecraft are somewhat similar.


Africa’s space industry has been slow to take off, but it’s predicted to skyrocket in the next few years.
Since the continent’s first satellite launched more than 20 years ago, 44 have been sent into orbit by 13 African countries, according to consultancy Space in Africa. It says a further 125 are being developed by 23 countries, all expected to launch before 2025.
The payoff could be substantial. A 2021 report by the World Economic Forum estimates that data collected from space could unlock $2 billion a year in benefits for Africa.
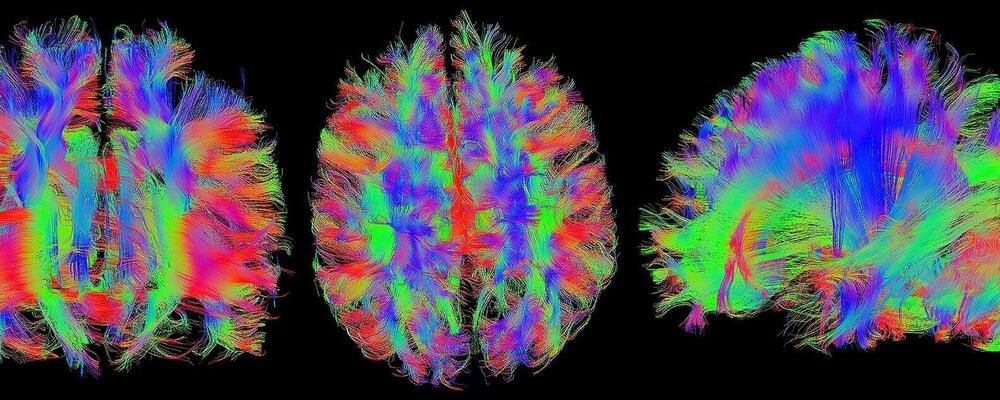
Science is examining the brain’s neural activity for applications ranging from innovative therapies for brain-related injuries and disease to computational learning architectures for artificial intelligence and deep neural networks.
A research team has developed a tool that lets researchers see more of a live mouse’s brain, to make discoveries that can advance research into the neural circuit mechanisms that form the underlying behavior of the human brain. The tool overcomes the drawback of traditional brain probes—the small amount of tissue they can access, which limits their ability to image neurons of interest.
The innovation is to insert an imaging probe with side-viewing capabilities into a previously inserted optically matched channel—an ultrathin-wall glass capillary—to convert deep brain imaging into endoscopic imaging. The operator can freely rotate the probe to image different brain regions, getting a 360-degree view for imaging along the entire length of the inserted probe. This large-volume imaging enables an increase of about 1,000 times in tissue access volume, compared with what is available for imaging at the tip of typical miniature imaging probes.
