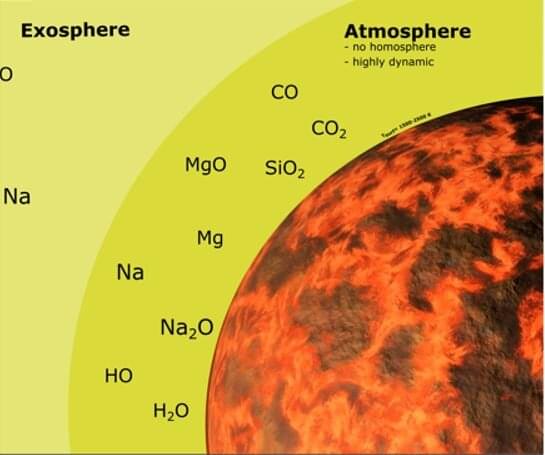
Mercury is a most unusual planet. The smallest planet in the solar system, and the closest planet to the sun, it is in a 3:2 spin resonance, slowly turning and experiencing scorching heat up to 430 degrees Celsius, and the night side frigid, down to-170 degrees Celsius. Due to its much larger iron-rich core compared to Earth, it has the second-highest average density in the solar system, just 1.5 percent below Earth’s. Despite its proximity to the sun, the surface of Mercury was, surprisingly, found to be rich in volatile elements such as sodium and sulfur.
Notably, the planet’s separation into an iron-rich core and rocky mantle (the geological region between the core and the crust) suggests Mercury had a magma ocean early in its formation. Like any liquid, this ocean would have evaporated, but in the case of Mercury, the temperatures were likely to have been so high that the vapor was not composed of water, but rock. In a new study published in The Planetary Science Journal, Noah Jäggi and colleagues modeled how the evaporation of the surface of this magma ocean would form an atmosphere and determined whether losses from the atmosphere could alter Mercury’s composition, addressing an open question of why moderately volatile elements like sodium have accumulated on Mercury’s surface. Their results were surprising, Jäggi, a graduate student at the University of Bern, told Phys.org.
Early planetary magma oceans aren’t unusual, explained Lindy Elkins-Tanton, director of the School of Earth and Space Exploration at Arizona State University. “We think all rocky planets have one or more—maybe several—magma oceans as they form. The impacts of accretion toward the end of planet formation are just that energetic; they will melt the planets to some depth.”