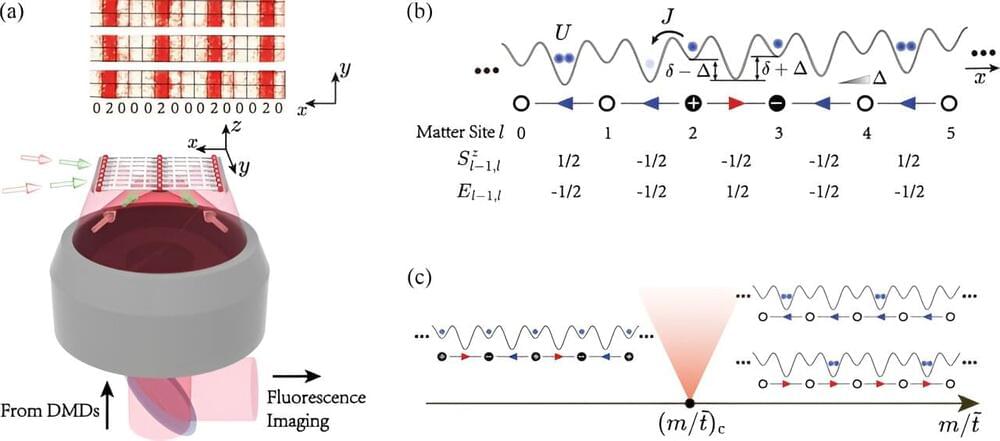
Researchers from the University of Science and Technology of China(USTC) of the Chinese Academy of Sciences (CAS) have developed an ultra-cold atom quantum simulator to study the relationship between the non-equilibrium thermalization process and quantum criticality in lattice gauge field theories. The research was led by Pan Jianwei and Yuan Zhensheng, in collaboration with Zhai Hui from Tsinghua University and Yao Zhiyuan from Lanzhou University.
Their findings reveal that multi-body systems possessing gauge symmetry tend to thermalize to an equilibrium state more easily when situated in a quantum phase transition critical region. The results were published in Physical Review Letters.
Gauge theory and statistical mechanics are two foundational theories of physics. From the Maxwell’s equations of classical electromagnetism to quantum electrodynamics and the Standard Model, which describe the interactions of fundamental particles, all adhere to specific gauge symmetries. On the other hand, statistical mechanics connects the microscopic states of large ensembles of particles (such as atoms and molecules) to their macroscopic statistical behaviors, based on the principle of maximum entropy proposed by Boltzmann and others. It elucidates, for instance, how the energy distribution of microscopic particles affects macroscopic quantities like pressure, volume, or temperature.