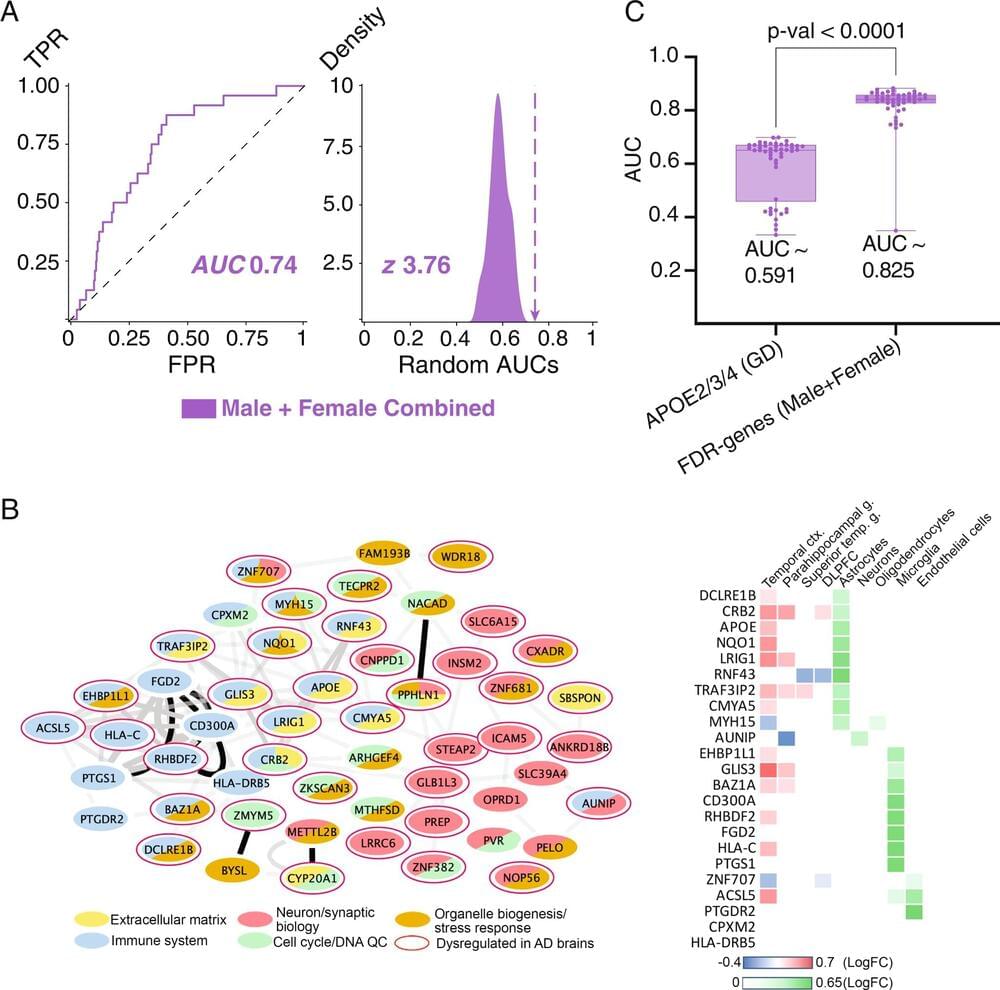
Alzheimer’s disease (AD) is a complex neurodegenerative illness with genetic and environmental origins. Females experience faster cognitive decline and cerebral atrophy than males, while males have greater mortality rates. Using a new machine-learning method they developed called “Evolutionary Action Machine Learning (EAML),” researchers at Baylor College of Medicine and the Jan and Dan Duncan Neurological Research Institute (Duncan NRI) at Texas Children’s Hospital have discovered sex-specific genes and molecular pathways that contribute to the development and progression of this condition. The study was published in Nature Communications.
“We have developed a unique machine-learning software that uses an advanced computational predictive metric called the evolutionary action (EA) score as a feature to identify genetic factors that influence AD risk separately in males and females,” Dr. Olivier Lichtarge, MD, Ph.D., professor of biochemistry and molecular biology at Baylor College of Medicine, said. “This approach lets us exploit a massive amount of evolutionary data efficiently, so we can now probe with greater accuracy smaller cohorts and identify genes involved in sex-specific differences in AD.”
EAML is an ensemble computational approach that includes nine machine learning algorithms to analyze the functional impact of non-synonymous coding variants, defined as DNA mutations that affect the structure and function of the resulting protein, and estimates their deleterious effect on biological processes using the evolutionary action (EA) score.


















