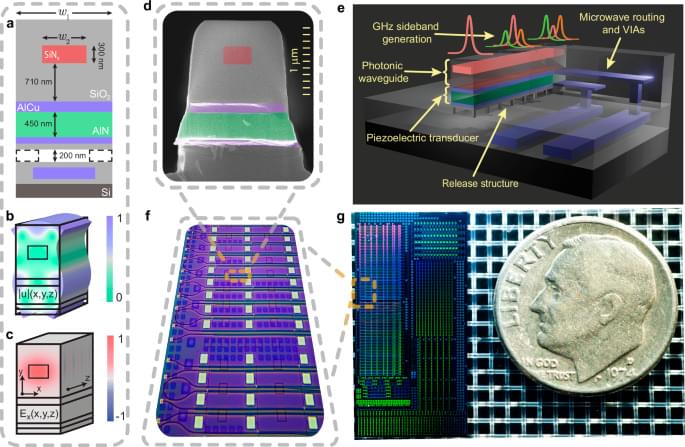
Scientists have captured an unprecedented, real-time view of influenza viruses as they move across and slip inside human cells. The footage reveals that cells are far from passive targets and instead push and pull against the virus in a surprisingly active struggle. Viewing Influenza Infection W.
Get the latest international news and world events from around the world.
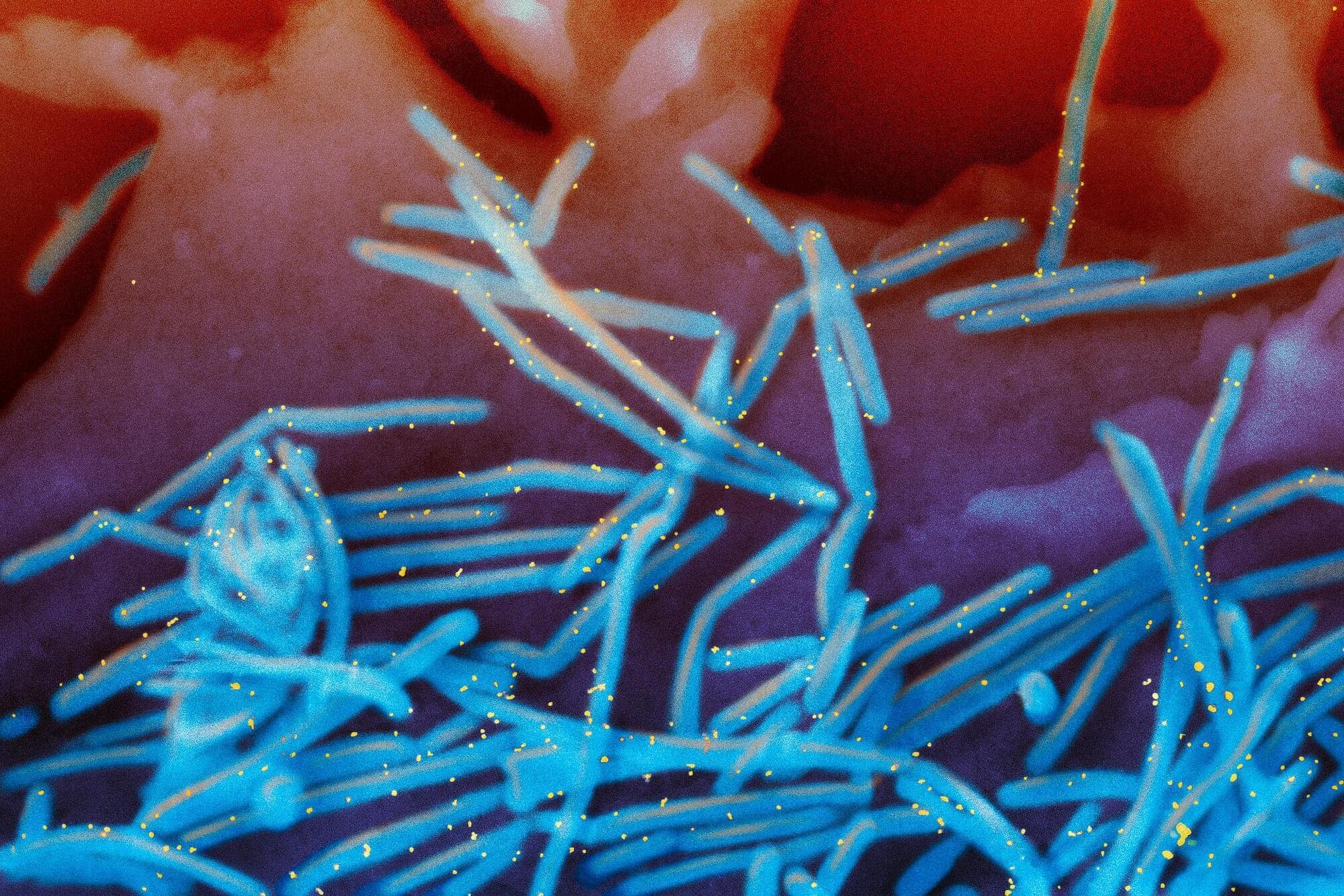
Winter virus season so far is not too bad, but doctors worry about suffering to come
It may feel like you are surrounded by sniffles and coughs, but flu season activity is still low in many parts of the U.S.
New government data posted Friday shows that as of last week, flu activity was high in four states—Colorado, Louisiana, New Jersey and New York—and minimal or low in most others. Severity indicators are increasing but are still within the boundaries of a “mild” season, said officials at the U.S. Centers for Disease Control and Prevention.
A number of diseases tend to peak in the winter, thanks to indoor gatherings that help germs spread. The list includes not only colds and flu but also norovirus—a highly infectious cause of vomiting and diarrhea. Norovirus cases have generally been trending up in the last month.


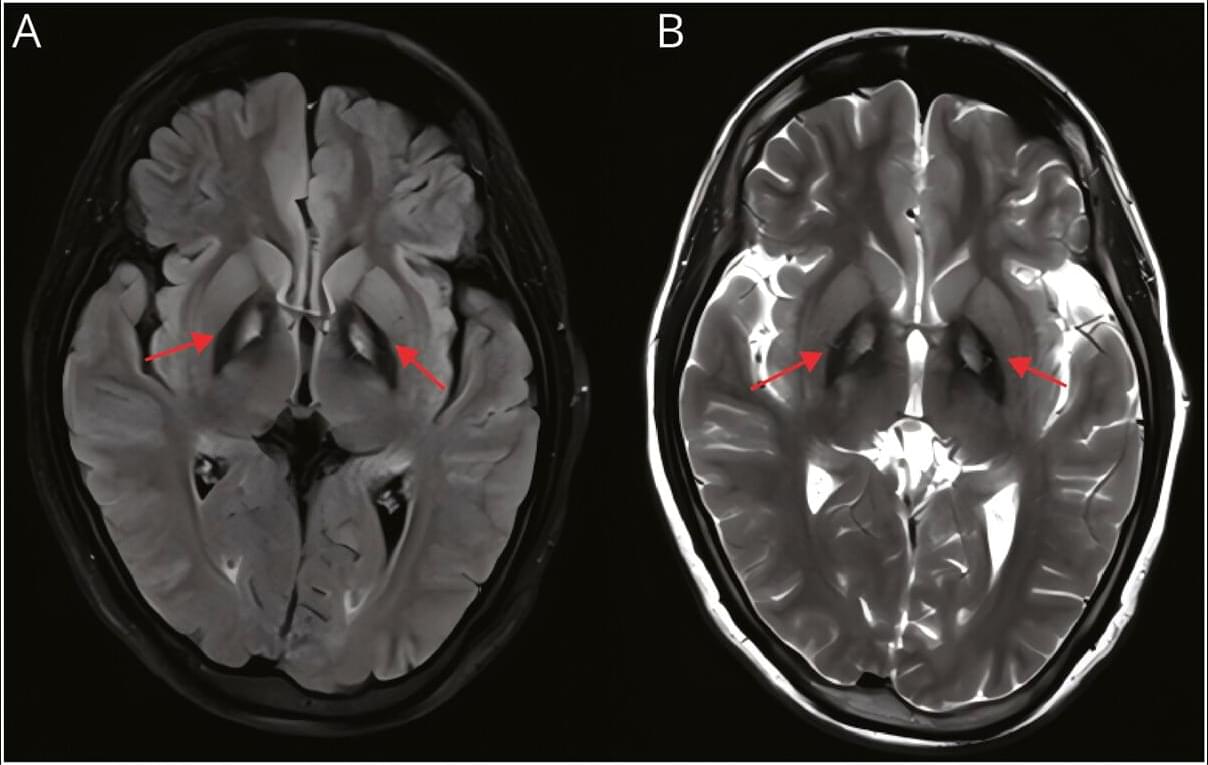
Video NeuroImage: Stereotypic Motor Behaviors in a Patient With Pantothenate Kinase–Associated Neurodegeneration
A 24-year-old woman with pantothenate kinase–associated neurodegeneration (PKAN) presented with a 5-year history of psychiatric symptoms followed by prominent stereotypic motor behaviors, including repetitive touching of her mouth and leg, object manipulation, and tip-toe walking (Video 1). Examination revealed severe depression and anxiety, mild speech dysfluency, and the stereotypic movements. Previous symptomatic treatments provided limited benefit. Brain magnetic resonance imaging demonstrated the pathognomonic “eye-of-the-tiger” sign, indicative of iron deposition in the bilateral globus pallidus (Figure). Genetic testing identified compound heterozygous variants in the PANK2 gene: a known pathogenic variant (c.401AG) and a novel likely pathogenic variant (c.1465CG).
This Photonic AI Chip is the FUTURE of Computer Vision
This AI chip doesn’t use electricity to compute — it uses light.
FlexiSpot is having mega sales now! Use my code “CODEOLENCES” to get EXTRA $30 off on the E7 Pro standing desk! If you’re shopping on a budget, the FlexiSpot premium E7 is a great option. It would be greatly appreciated if you could leave a note saying “Codeolences” at checkout. FlexiSpot E7 Pro standing desk:
USA: https://bit.ly/497nWv1
CAN: https://bit.ly/4iTaKNO
▀▀▀
Engineers at the University of Pennsylvania have built a photonic neural network capable of classifying nearly 2 billion images per second, operating at speeds millions of times faster than today’s electronic computer vision systems.
In this video, we explore how photonic neural networks work, why traditional image recognition is so computationally expensive, and how light-based hardware could overcome fundamental limits of GPUs and silicon. We go over how convolution layers, weighted sums, and activation functions are implemented directly on a photonic chip — without memory, clock cycles, or digital logic.
⚛️⚛️⚛️