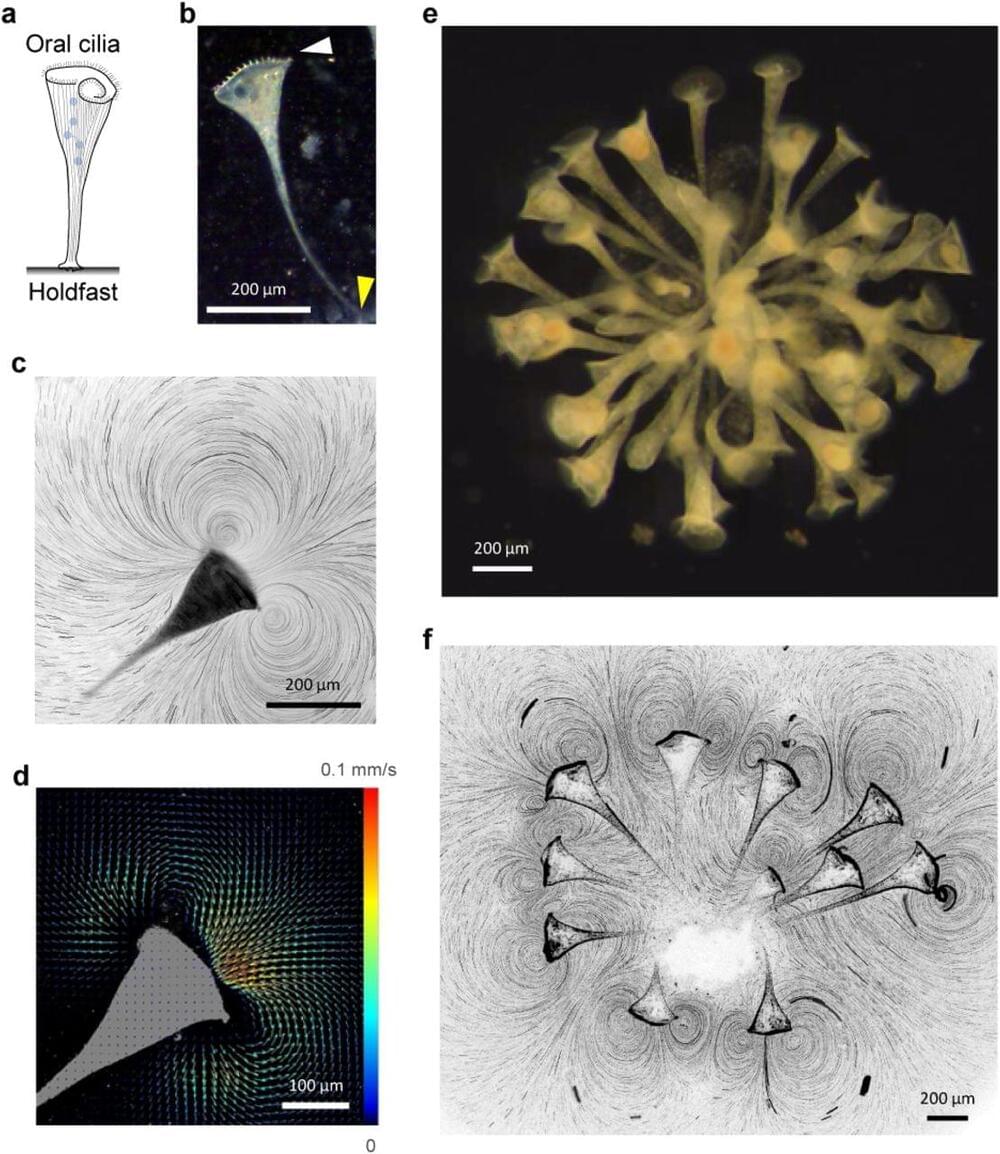
Evolution of multicellularity from early unicellular ancestors is arguably one of the most important transitions since the origin of life1,2. Multicellularity is often associated with higher nutrient uptake3, better defense against predation, cell specialization and better division of labor4. While many single-celled organisms exhibit both solitary and colonial existence3,5,6, the organizing principles governing the transition and the benefits endowed are less clear. Using the suspension-feeding unicellular protist Stentor coeruleus, we show that hydrodynamic coupling between proximal neighbors results in faster feeding flows that depend on the separation between individuals. Moreover, we find that the accrued benefits in feeding current enhancement are typically asymmetric– individuals with slower solitary currents gain more from partnering than those with faster currents. We find that colony-formation is ephemeral in Stentor and individuals in colonies are highly dynamic unlike other colony-forming organisms like Volvox carteri 3. Our results demonstrate benefits endowed by the colonial organization in a simple unicellular organism and can potentially provide fundamental insights into the selective forces favoring early evolution of multicellular organization.
Suspension-feeding unicellular protists inhabit a fluid world dominated by viscous forces that limit prey transport for feeding 3,7,8. Using either flagella or cilia, many of these organisms generate microcurrents that actively transport dissolved nutrients and smaller prey critical for their nutrition 3,9,10. A protist’s ability to favorably alter its feeding current so as to enhance feeding rate would therefore be beneficial to its survival. Can colony formation enable unicellular protists to enhance their feeding flows? Colonial protists have been suggested to generate stronger flows by combining individual feeding microcurrents of neighboring colony members. Colony forming protists can broadly be classified into two categories depending on presence (or absence) of physical linkages between colony members.