We have very specific predictions for how particles ought to decay. When we look at B-mesons all together, something vital doesn’t add up.

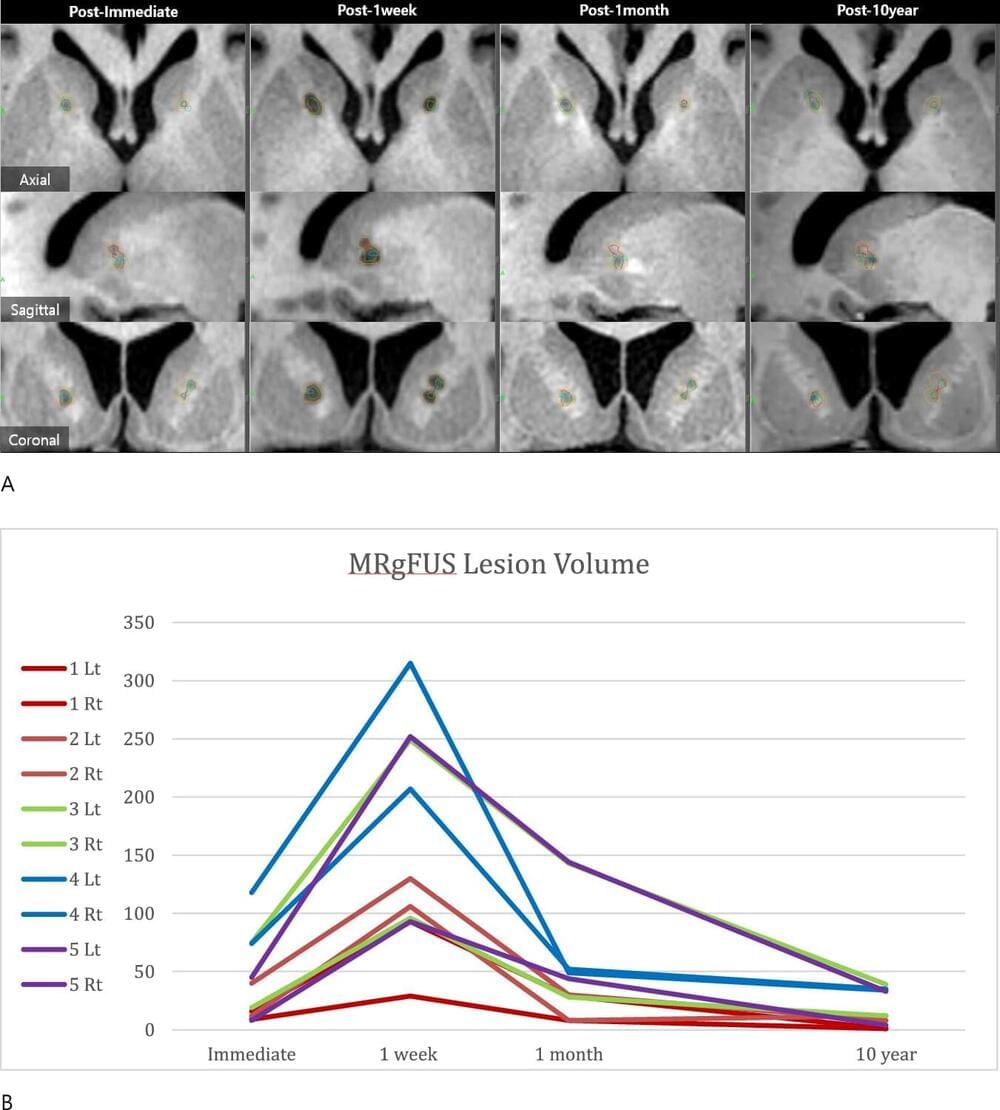
When conventional non-invasive treatments for psychiatric diseases fail, clinicians inevitably have to consider brain surgery. However, brain surgery for psychiatric diseases has long been taboo among the general public due to the infamous history of lobotomy. Thankfully, advancements in brain surgery in recent years are changing the narrative.
Bilateral capsulotomy, more commonly known as deep brain stimulation, is a form of brain surgery that has been garnering attention in treating treatment-resistant or refractory obsessive-compulsive disorder (OCD). Patients with refractory OCD experience a debilitating degree of repetitive behaviors and thoughts that they are unable to control, thus downgrading their quality of life.
A group of researchers from South Korea demonstrated that a novel non-invasive bilateral capsulotomy called magnetic resonance-guided focused ultrasound (MRgFUS) capsulotomy is efficacious and safe in treating refractory OCD for up to two years. MRgFUS capsulotomy non-invasively and precisely ablates tissues in the brain region of interest. However, the sustained efficacy of this treatment option was unclear.
A groundbreaking study just revealed AI outperforming human doctors at medical diagnosis — but before you panic, this could be the best news yet for healthcare.
This hits personally for me. From my kiddo’s misdiagnosed case of hives to my own health struggles with multiple doctors, I’ve seen firsthand why we need AI to empower (not replace) medical professionals. I’m sure I’m not the only one.
In this video, we’ll explore:
-The shocking study results (90% AI accuracy vs 74% human doctors)
–Why this means more human connection, not less.
–How AI could transform patient care for the better.
–The real reason doctors aren’t fully utilizing AI yet.
The future of healthcare isn’t AI vs doctors — it’s both working together to provide better care than either could alone. Let’s dive into what this means for you and your family’s healthcare.

Boosted by China’s rapid development pace in artificial intelligence (AI) technologies, more companies have noted the huge business potential in AI companionship sectors, as simulated AI pets with adorable appearances gaining increasing popularity among Chinese consumers.
Zhang Yi, CEO of the iiMedia Research Institute, told the Global Times on Sunday that consumers’ demand for emotional support and the capability of current AI technologies offer this type of products greater business potential.
A Beijing-based student in her 20’s surnamed Zhang, who is also an AI technology fan, told the Global Times on Sunday that she bought “Boo Boo,” a simulated AI robotic pet developed by Hangzhou-based Genmoor Technology.
Researchers have developed a liquid ink that can be printed directly onto the scalp to monitor brain activity, offering a less intrusive alternative to traditional EEG setups.
This ink enables the creation of e-tattoos that accurately track brainwaves and maintain connectivity over extended periods. These innovations could drastically change the application of brain-computer interface technologies, making them more comfortable and efficient for users.
Innovative liquid ink for brain activity monitoring.

The dream of many – to try the taste through a monitor – is getting closer.
A team of biomedical engineers and virtual reality experts has developed a groundbreaking lollipop-shaped interface that simulates taste in virtual reality.
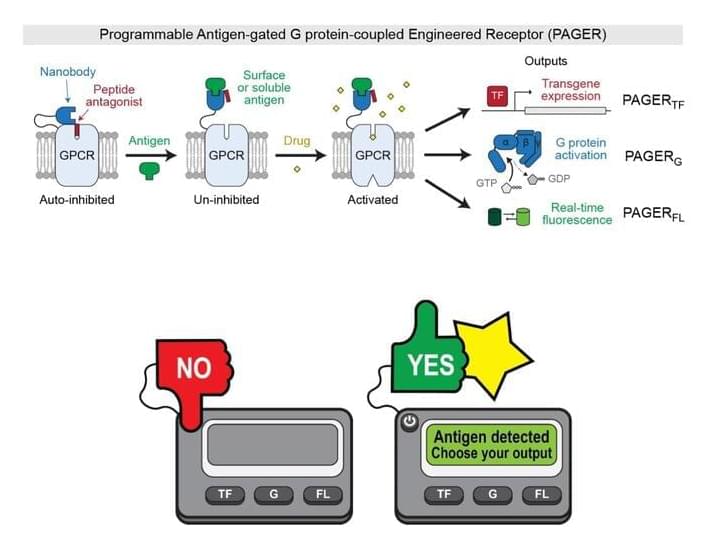
A basic function of cells is that they act in response to their environments. It makes sense, then, that a goal of scientists is to control that process, making cells respond how they want to what they want.
One avenue for this ambition is cell receptors, which function like ignition slots on a cell, requiring keys—such as specific hormones, drugs, or antigens—to start up specific cellular activities. There are already synthetic receptors that give us some control over this sequence of events, most famously the chimeric antigen receptors used in CAR-T cell cancer therapy. But existing synthetic receptors are limited in the variety of keys they can accept and the activities they can trigger.
Now, detailed in a paper published in Nature, Stanford researchers have developed a new synthetic receptor that accommodates a broader range of inputs and produces a more diverse set of outputs.

Scientists in China have claimed a breakthrough that might completely change how we store energy by turning waste oil into a formidable substance for energy storage.
As the world grapples with increasing power demand, supercapacitors are becoming more popular because of their quick charging and discharging times, which makes them perfect for high-performance applications.
The researcher’s novel method provides a sustainable way to make these supercapacitors while addressing waste management and energy storage challenges, according to a press release by the Chinese Academy of Sciences (CAS).
Multisectoral approaches for combating antimicrobial resistance — dr. amal al-maani — director general, diseases surveillance & control, ministry of health oman.
Dr. Amal Al-Maani, MD is Director General for Diseases Surveillance and Control at the Ministry of Health of Oman (https://moh.gov.om/en/hospitals-direc…), senior consultant in pediatric infectious diseases in the Sultanate, and is the focal point for the Global Antimicrobial Resistance (AMR) Surveillance System (GLASS) and is responsible for Oman national surveillance system for AMR (OMASS) and the national Infection Prevention and Control (IPC) program.
Dr. Al-Maani completed her medical degree from Sultan Qaboos University, Oman and passed the London School diploma in tropical Medicine and Hygiene (DTM\&H) during her internship period. Followed by her postgraduate training at the University of Toronto, she achieved her fellowship in pediatric infectious diseases from the Royal College of Physicians and Surgeons, Canada. She has the Certificate In Infection Control from the Certification Board of Infection Control \& Epidemiology, a certificate in global health from Dalla Lana School of Public Health the Centre for International Health in the University of Toronto (UFT), and the Patient Safety \& Quality Improvement certificate from the center for patient safety in UFT.
Dr. Al-Maani has participated in many national and International Conferences and presented many papers. She received Dr Susan King Award at the Canadian AMMI conference 2011 and in 2021 the WHA Sasakawa health development award for her work in AMR and IPC. She published many papers in the field of infectious diseases and infection control with a focus on Antimicrobial resistance and emerging resistant pathogens. She had been a co-author in multiple positional statements for the International Society for Infectious Diseases (ISID) group in infection control, including most recently about the Global Antimicrobial Stewardship with a Focus on Low-and Middle-Income Countries and on the Prevention of Clostridioides.
#AntimicrobialResistance #AMR #AmalAlMaani #DiseasesSurveillance #MinistryOfHealth #Oman #SultanQaboosUniversity #WHO #WorldHealthOrganization #OneHealth #Antibiotics #Vaccines #TropicalMedicine #Hygiene #VancomycinResistantEnterococcus #MethicillinResistantStaphylococcus #ProgressPotentialAndPossibilities #IraPastor #Podcast #Podcaster #ViralPodcast #STEM #Innovation #Technology #Science #Research
Meta might yet teach its AI to more consistently show the right posts at the right time. Still, there’s a bigger lesson it could learn from Bluesky, though it might be an uncomfortable one for a tech giant to confront. It’s that introducing algorithms into a social feed may cause more problems than it solves—at least if timeliness matters, as it does with any service that aspires to scoop up disaffected Twitter users.
For a modern social network, Bluesky stays out of your way to a shocking degree. (So does Mastodon; I’m a fan, but it seems to be more of an acquired taste.) Bluesky’s primary view is “Following”—the most recent posts from the people you choose to follow, just as in the golden age of Twitter. (Present-day Twitter and Threads have equivalent views, but not as their defaults.) Starter Packs, which might be Bluesky’s defining feature, let anyone curate a shareable list of users. You can follow everyone in one with a single click, or pick and choose, but either way, you decide.