Meta Platforms on Friday said it will invest $600 billion in U.S. infrastructure and jobs over the next three years, including artificial intelligence data centers, as the social media giant races to build infrastructure to power its AI ambitions.


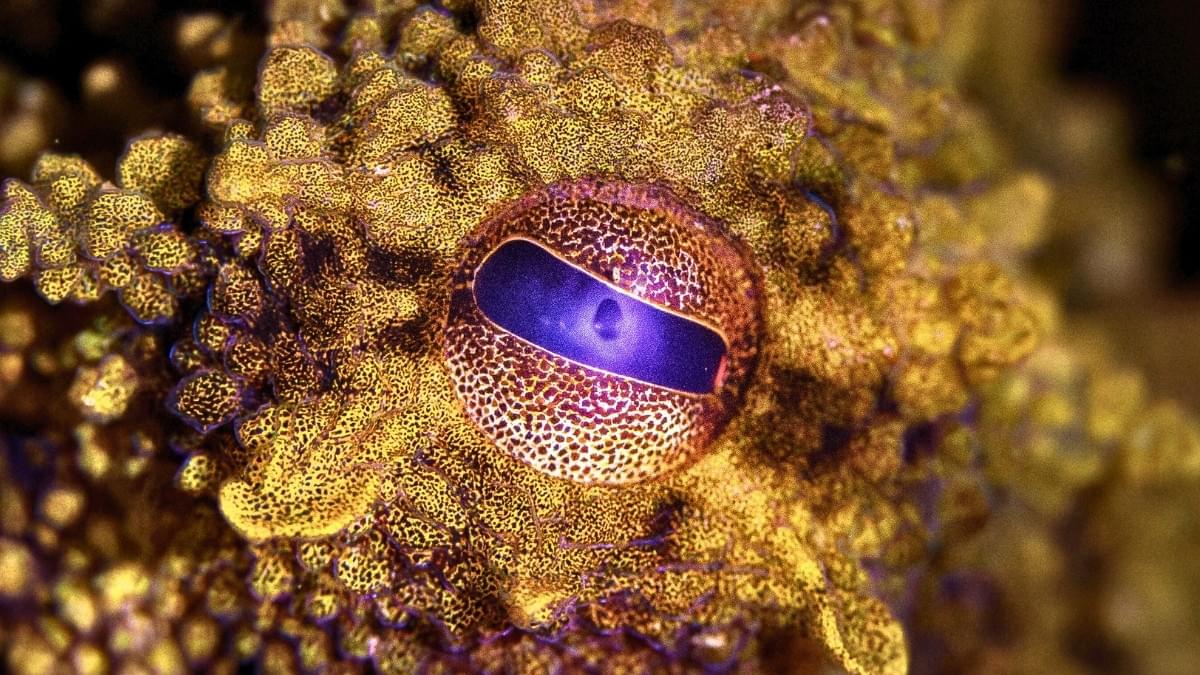
Octopuses and other cephalopods are masters of camouflage, thanks largely to color-changing skin that can help them seemingly vanish into the background. Now, researchers report a big step towards being able to recreate their superpower.
A team led by UC San Diego was able to mass-produce a key pigment, xanthommatin, that occurs in the psychedelic skin of many cephalopods. Until now, xanthommatin has proven impractical to collect from animals or make in a lab.
The researchers technically didn’t make the pigment. They bioengineered bacteria to make it, coaxing microbes to not only produce this rare substance, but to do so with unprecedented efficiency, yielding up to 1,000 times more xanthommatin than previous methods.

The study points to a few other benefits: Modern antivenoms don’t address the tissue damage that can be wrought by snake venom, but the nanobodies in the new product seemed to decrease tissue injury in mice, even with delayed treatment. And since nanobodies are less likely to cause serious immune reactions, per the statement, clinicians could theoretically administer the new antivenom before the appearance of clear symptoms, instead of waiting in an attempt to avoid severe side effects.
Still, because the experiments were performed on lab animals, not humans, the new antivenom is nowhere near commercial availability—first, the concept must be proven in human subjects. As such, the team is working on improving the antivenom and securing more funding.
“We have both a moral and global responsibility to contribute to solving this problem,” Laustsen-Kiel says in the statement. “Our antivenom has the potential to fundamentally change how snakebites are treated around the world.”

A new study shows children and young people face long-lasting and higher risks of rare heart and inflammatory complications after COVID-19 infection, compared to before or without an infection. Meanwhile, the COVID-19 vaccination was only linked to a short-term higher risk of myocarditis and pericarditis.
The study is the largest of its kind in this population, and is published in The Lancet Child and Adolescent Health. It was led by scientists at the Universities of Cambridge and Edinburgh, and University College London, with support from the BHF Data Science Center at Health Data Research UK.
Principal author Dr. Alexia Sampri, University of Cambridge, said, “Our whole-population study during the pandemic showed that although these conditions were rare, children and young people were more likely to experience heart, vascular or inflammatory problems after a COVID-19 infection than after having the vaccine—and the risks after infection lasted much longer.”
The Lunar Module’s Descent Propulsion System (DPS) was the first engine in history that could throttle continuously in deep space — a breakthrough that made Apollo’s lunar landing possible. This engine had to ignite once, vary its thrust smoothly from 10 to 100 percent, avoid combustion instability, and hold steady while the LM hovered just feet above the Moon.
In this video, we explore the real engineering behind the DPS: its hypergolic fuels, injector plate design, the early “chugging” instability problem, throttle control logic, and how the engine kept working even as Apollo 11 pushed it to its limits.
If you enjoy deep dives into Apollo engineering, this one’s for you.
🚀 Every like, comment, and share helps keep Apollo’s engineering story alive.
If you liked this video, please share it with a friend and leave a comment below — it really helps the channel grow.
🚀 New Apollo episodes every week!
📘 Recommended Reading for Space Enthusiasts.
Explore the real stories, engineering, and people behind the Apollo Program — these are the best books to deepen your knowledge:

Placing materials under extremely strong magnetic fields can give rise to unusual and fascinating physical phenomena or behavior. Specifically, studies show that under magnetic fields above 100 tesla (T), spins (i.e., intrinsic magnetic orientations of electrons) and atoms start forming new arrangements, promoting new phases of matter or stretching a crystal lattice.
One physical effect that can take place under these extreme conditions is known as magnetostriction. This effect essentially prompts a material’s crystal structure to stretch out, shrink or deform.
When magnetic fields above 100 T are produced experimentally, they can only be maintained for a very short time, typically for only a few microseconds. This is because their generation poses great stress on the wires used to produce the fields (i.e., coils), causing them to break almost immediately.

The last decade has seen incredible progress in machine learning (ML), primarily driven by powerful neural network architectures and the algorithms used to train them. However, despite the success of large language models (LLMs), a few fundamental challenges persist, especially around continual learning, the ability for a model to actively acquire new knowledge and skills over time without forgetting old ones.
When it comes to continual learning and self-improvement, the human brain is the gold standard. It adapts through neuroplasticity — the remarkable capacity to change its structure in response to new experiences, memories, and learning. Without this ability, a person is limited to immediate context (like anterograde amnesia). We see a similar limitation in current LLMs: their knowledge is confined to either the immediate context of their input window or the static information that they learn during pre-training.
The simple approach, continually updating a model’s parameters with new data, often leads to “catastrophic forgetting” (CF), where learning new tasks sacrifices proficiency on old tasks. Researchers traditionally combat CF through architectural tweaks or better optimization rules. However, for too long, we have treated the model’s architecture (the network structure) and the optimization algorithm (the training rule) as two separate things, which prevents us from achieving a truly unified, efficient learning system.

