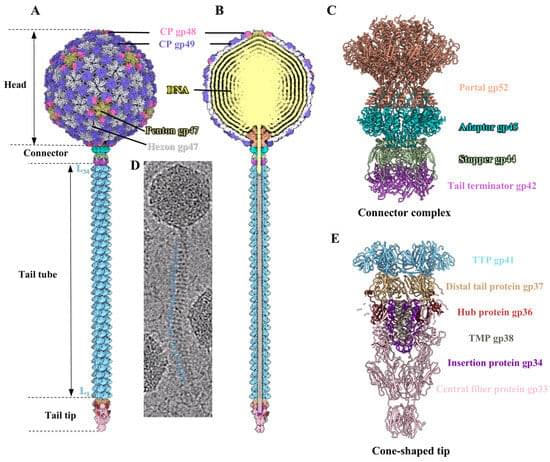
The difference between the brains of children who read books (left picture) and screen time (right picture) over 1 hour. Early childhood, screen time over 60 minutes, are vulnerable to emotional and focus disorders. Increasing the duration of screen time reduces brain connectivity in the language, visual and intelligence centres compared to reading books.
The school bell rang long ago, but Danny is still sitting in his chair, trying to finish copying from the board. “Why is this process so hard? Why does it take me so much longer to read than it takes my friends?” Danny is frustrated. The more he tries to read faster, the harder it is for him to understand what he is reading. Around the time when he finally finishes copying, his friends come back to the class from the break. Like 10–15% of the children in the world, Danny has dyslexia. Dyslexia is defined as difficulty in reading accurately or quickly and, most of the time; it affects the person’s ability to understand what is read and to spell words correctly. The reading difficulty continues into adulthood and does not disappear, even though most adults with dyslexia find ways to “bypass” this difficulty, sometimes using text-to-speech software. Children and adults with dyslexia have different brain activity than do people who are good readers. They have lower activity in the brain area responsible for vision and identification of words [ 1, 2 ] and in another brain area responsible for attention and recognition of errors during reading [ 3 ]. A question could then be asked: is this reading difficulty strange or is it actually the ability to read that is magical? How did the human brain learn to read? And does the daily use of technology, which sometimes “bypasses” the need to make an effort to read, help us to learn to read or make it more difficult? This article will discuss these subjects.
Reading is a relatively new human ability—about 5,000 years old. The Egyptians were among the first to use symbols to represent words within a spoken language, and they used drawings to transmit ideas via writing. As difficult as it is to draw each word in a language, it is still much easier to understand Egyptian hieroglyphs than to figure out what is written in an unfamiliar language. Today, 5,000 years later, we expect each child in first grade to immediately understand that the lines and circles that form letters have a unique sound corresponding to them. To do that, the brain has to rely on neural networks that were designed to perform other tasks, such as seeing, hearing, language comprehension, speech, attention, and concentration [ 4 ] (see Figure 1).