Learn how scientists found a way to turn mushrooms into computer chips and how these living computers can make the future of technology more sustainable.



For the past 250 years, people have mined coal industrially in Pennsylvania, U.S… By 1830, the city of Pittsburgh was using more than 400 tons of the fossil fuel every day. Burning all that coal has contributed to climate change. Additionally, unremediated mines—especially those that operated before Congress passed regulations in 1977 —have leaked environmentally harmful mine drainage. But that might not be the end of their legacy.
In research presented last week at GSA Connects 2025 in San Antonio, Texas, U.S., Dr. Dorothy Vesper, a geochemist at West Virginia University, found that those abandoned mines pose another risk: continuous CO2 emissions from water that leaks out even decades or centuries after mining stops.

In the race to make solar energy cheaper and more efficient, a team of UNSW Sydney scientists and engineers have found a way to push past one of the biggest limits in renewable technology.
Singlet fission is a process where a single particle of light—a photon—can be split into two packets of energy, effectively doubling the electrical output when applied to technologies harnessing the sun.
In a study appearing in ACS Energy Letters, the UNSW team—known as “Omega Silicon”—showed how this works on an organic material that could one day be mass-produced specifically for use with solar panels.
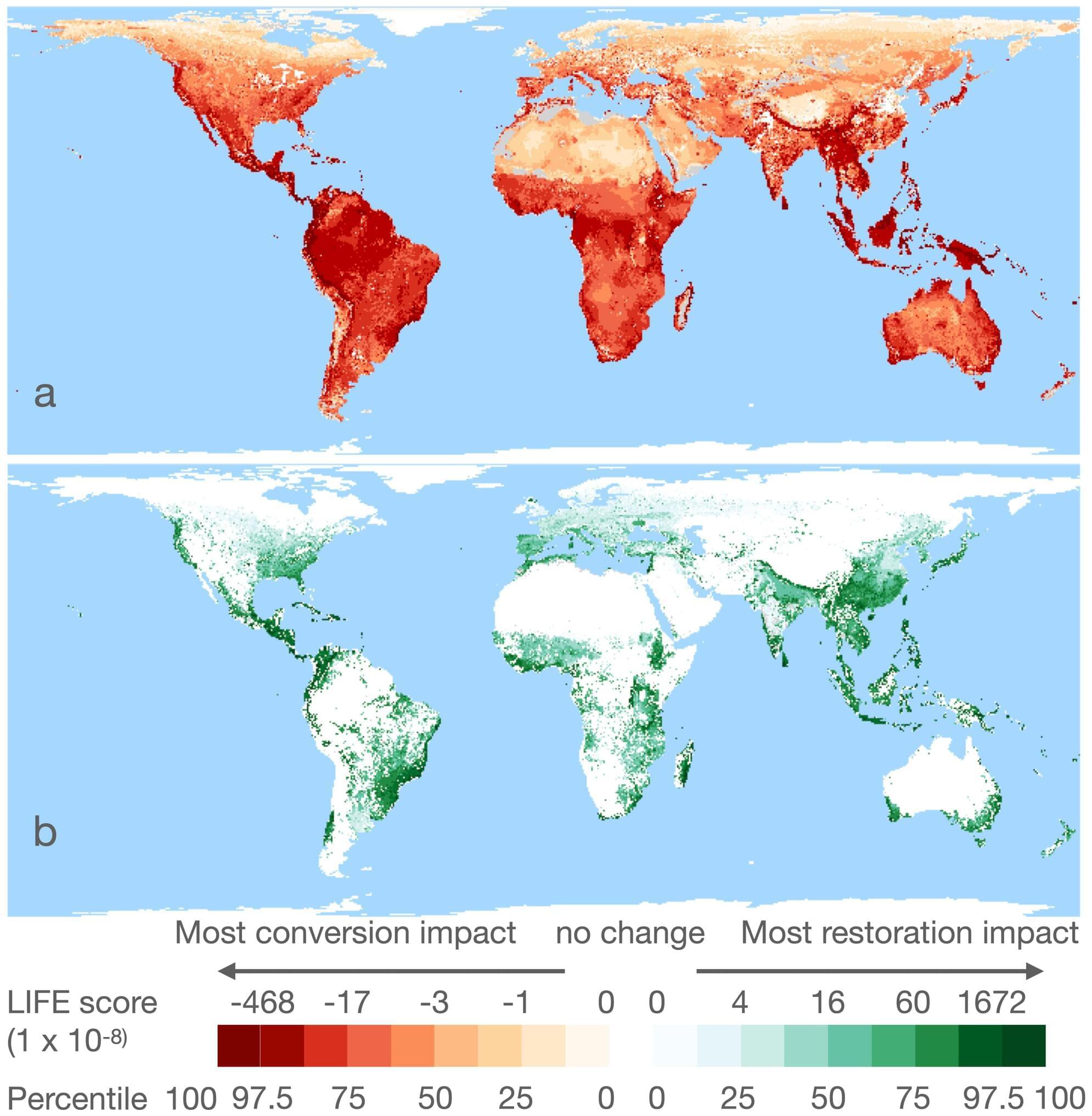
University of Cambridge researchers have developed a new way to measure the impact of our food production on other species’ survival around the world.
It reveals that between 700 and 1,100 species of vertebrate are likely to go extinct in the next 100 years, if global land-use for agriculture does not change. This figure does not account for future population growth, and is probably a huge underestimate.
By considering the productivity of any piece of land, the team can figure out the “per kilogram impact” of each commodity per year on biodiversity.

Scientists have long sought to make semiconductors—vital components in computer chips and solar cells—that are also superconducting, thereby enhancing their speed and energy efficiency and enabling new quantum technologies. However, achieving superconductivity in semiconductor materials such as silicon and germanium has proved challenging due to difficulty in maintaining an optimal atomic structure with the desired conduction behavior.
In a paper published in the journal Nature Nanotechnology, an international team of scientists reports producing a form of germanium that is superconducting—able to conduct electricity with zero resistance, which allows currents to flow indefinitely without energy loss, resulting in greater operational speed that requires less energy.
“Establishing superconductivity in germanium, which is already widely used in computer chips and fiber optics, can potentially revolutionize scores of consumer products and industrial technologies,” says New York University physicist Javad Shabani, director of NYU’s Center of Quantum Information Physics and the university’s newly established Quantum Institute, one of the paper’s authors.
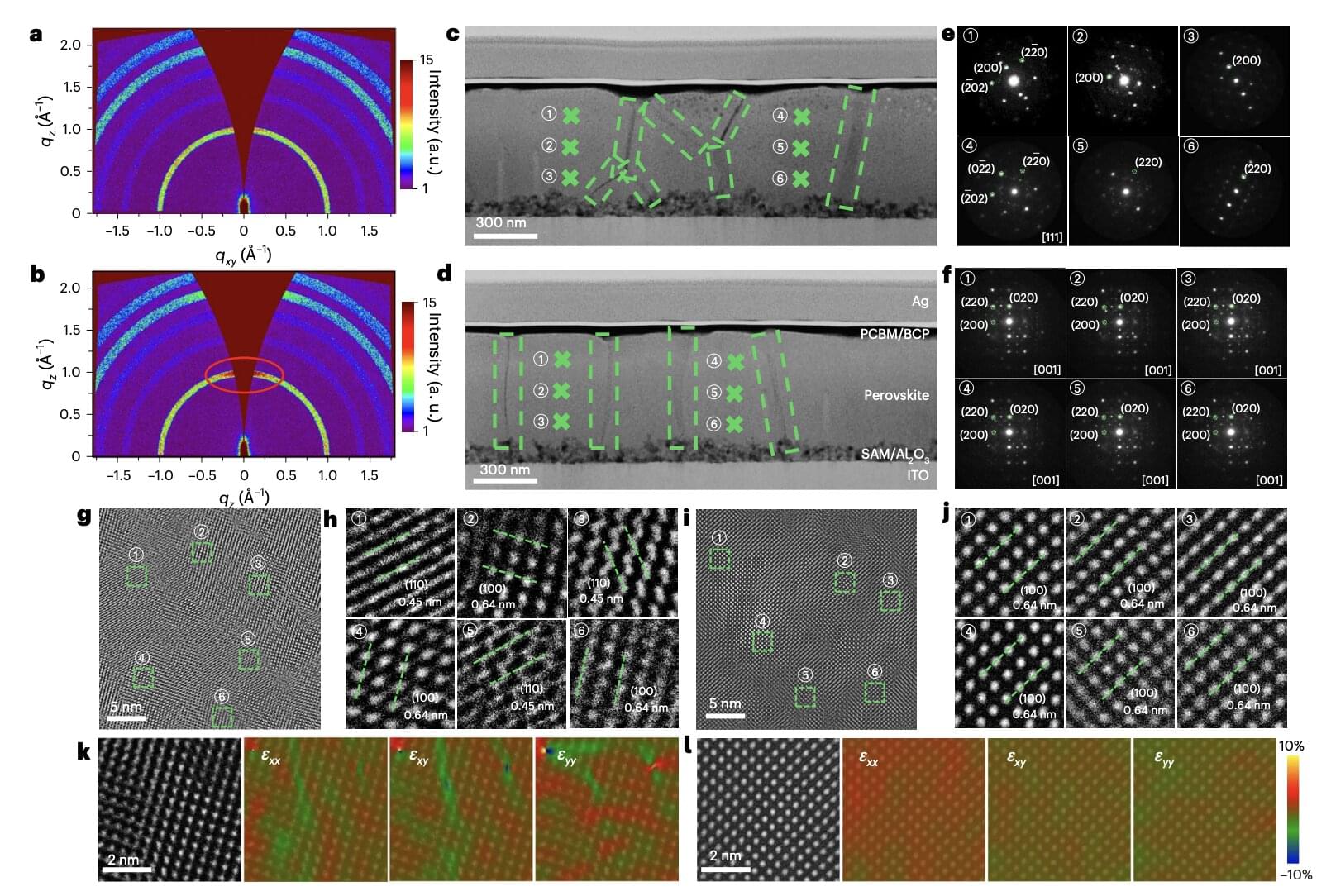
Solar cells, devices that can directly convert radiation emitted from the sun into electricity, have become increasingly widespread and are contributing to the reduction of greenhouse gas emissions worldwide. While existing silicon-based solar cells have attained good performances, energy engineers have been exploring alternative designs that could be more efficient and affordable.
Perovskites, a class of materials with a characteristic crystal structure, have proved to be particularly promising for the development of low-cost and energy-efficient solar energy solutions. Recent studies specifically highlighted the potential of inverted perovskite solar cells, devices in which the extraction charge layers are arranged in the reverse order compared to traditional designs.
Inverted perovskite solar cells could be more stable and easier to manufacture on a large-scale than conventional perovskite-based cells. Nonetheless, most inverted cells developed so far were found to exhibit low energy-efficiencies, due to the uncontrolled formation of crystal grains that can produce defects and adversely impact the transport of charge carriers generated by sunlight.
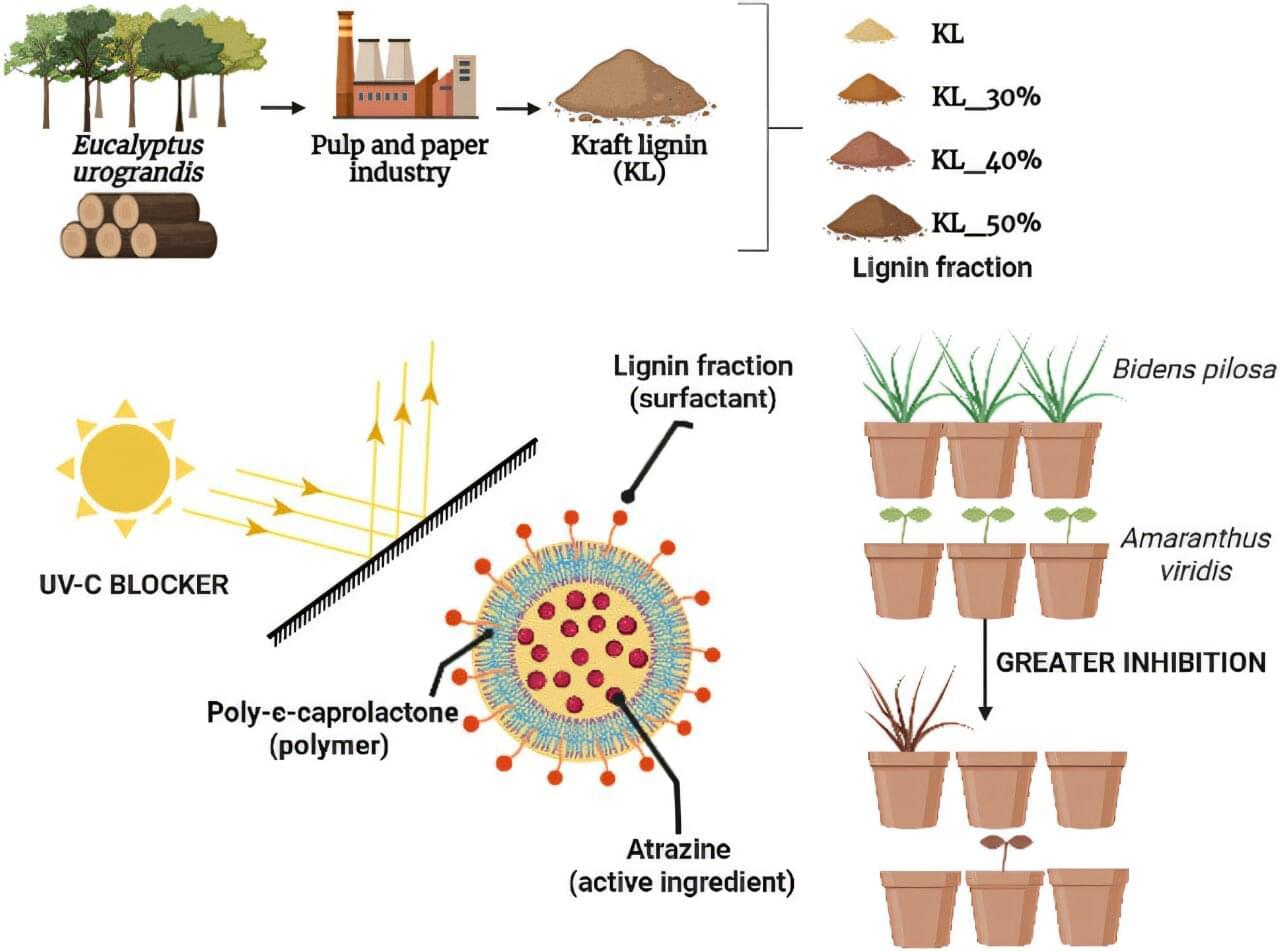
A recent study has shown that a fraction obtained from lignin, an organic polymer responsible for the rigidity of plant cell walls, was able to improve the performance of nanoparticles with herbicide.
The work is published in the journal ACS Sustainable Chemistry & Engineering and was recently featured on its cover.
The study was conducted by researchers from three research institutions in the state of São Paulo, Brazil: São Paulo State University (UNESP), the State University of Campinas (UNICAMP), and the Federal University of São Carlos (UFSCar).

“The ability to resolve the Gulf Stream and its dynamics properly, has been an open challenge for many years in oceanography,” said Dr. Ashesh Chattopadhyay.
How can AI be used to predict ocean forecasting? This is what a recent study published in the Journal of Geophysical Research Machine Learning and Computation hopes to address as a team of researchers investigated how AI can be used to predict short-and long-term trends in ocean dynamics. This study has the potential to help scientists and the public better understand new methods estimating long-term ocean forecasting, specifically with climate change increasing ocean temperatures.
For the study, the researchers presented a new AI-based modeling tool for predicting ocean dynamics for the Gulf of Mexico, which is a major trade route between the United States and Mexico. The goal of the tool is to build upon longstanding physics-based models that have traditionally been used for predicting ocean dynamics, including temperature and changes in temperature.
In the end, the researchers found that this new model demonstrates improved performance in predicting ocean dynamics, specifically for short-term intervals of 30 days, along with long-term intervals of 10 years. The team aspires to use this new tool for modeling ocean dynamics worldwide.
