Powering plant growth with solar panels instead of photosynthesis could be a more efficient way of using the Sun’s energy for food. But it’s not all good news.
Category: solar power
Scientists in Germany looked to eliminate the use of toxic solvents in the production of perovskite solar cells, replacing them with a more environmentally material called dimethyl sulfoxide (DMSO) which has so far proved difficult to integrate into processes suitable for large-scale production. The group demonstrated a scalable blade coating process using DMSO as the only solvent, and reached cell efficiencies close to those achieved using more toxic substances.
The average global price of solar kilowatt-hours fell 13% on 2020’s prices, as around two-thirds of the renewables capacity installed last year was cheaper than the lowest-cost fossil fuel alternative.
The first tandem perovskite-silicon solar cells to exceed 30% efficiency have been independently certified.
Lightweight and flexible perovskites are highly promising materials for the fabrication of photovoltaics. So far, however, their highest reported efficiencies have been around 20%, which is considerably lower than those of rigid perovskites (25.7%).
Researchers at Nanjing University, Jilin University, Shanghai Tech University, and East China Normal University have recently introduced a new strategy to develop more efficient solar cells based on flexible perovskites. This strategy, introduced in a paper published in Nature Energy, entails the use of two hole-selective molecules based on carbazole cores and phosphonic acid anchoring groups to bridge the perovskite with a low temperature-processed NiO nanocrystal film.
“We believe that lightweight flexible perovskite solar cells are promising for building integrated photovoltaics, wearable electronics, portable energy systems and aerospace applications,” Hairen Tan, one of the researchers who carried out the study, told TechXplore. “However, their highest certified efficiency of 19.9% lags behind their rigid counterparts (highest 25.7%), mainly due to defective interfaces at charge-selective contacts with perovskites atop.”
Photosynthesis uses a series of chemical reactions to convert carbon dioxide, water, and sunlight into glucose and oxygen. The light-dependent stage comes first, and relies on sunlight to transfer energy to plants, which convert it to chemical energy. The light-independent stage (also called the Calvin Cycle) follows, when this chemical energy and carbon dioxide are used to form carbohydrate molecules (like glucose).
A research team from UC Riverside and the University of Delaware found a way to leapfrog over the light-dependent stage entirely, providing plants with the chemical energy they need to complete the Calvin Cycle in total darkness. They used an electrolysis to convert carbon dioxide and water into acetate, a salt or ester form of acetic acid and a common building block for biosynthesis (it’s also the main component of vinegar). The team fed the acetate to plants in the dark, finding they were able to use it as they would have used the chemical energy they’d get from sunlight.
They tried their method on several varieties of plants and measured the differences in growth efficiency as compared to regular photosynthesis. Green algae grew four times more efficiently, while yeast saw an 18-fold improvement.
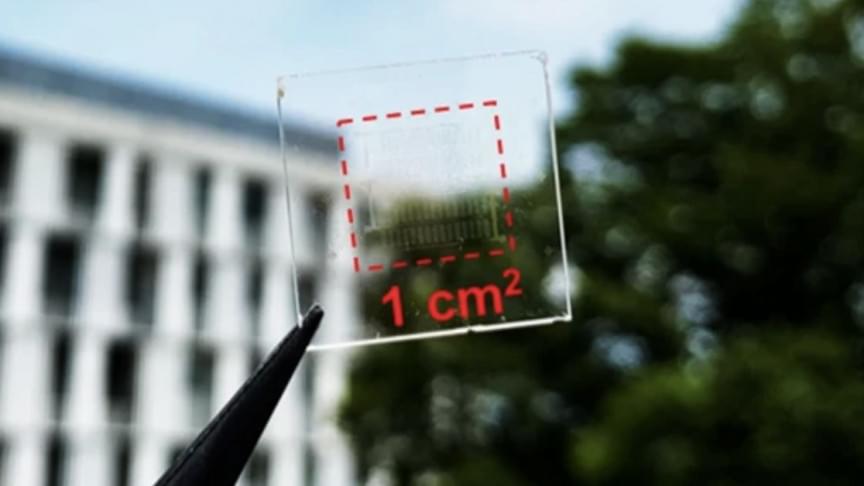
The dream of transforming windows into active power generators has just edged one step closer to realization.
A team of researchers from ARC Centre of Excellence in Exciton Science led by Professor Jacek Jasieniak from Monash University’s Department of Materials Science and Engineering has created perovskite cells with a conversion efficiency of 15.5 percent that allows more than 20 percent of visible light through, a press release states.
This improves the stability of solar windows while allowing more natural light in, which means the amount of visible light passing through the cells is remarkably now reaching glazing levels, increasing their potential for usage in a wide range of real-world applications.