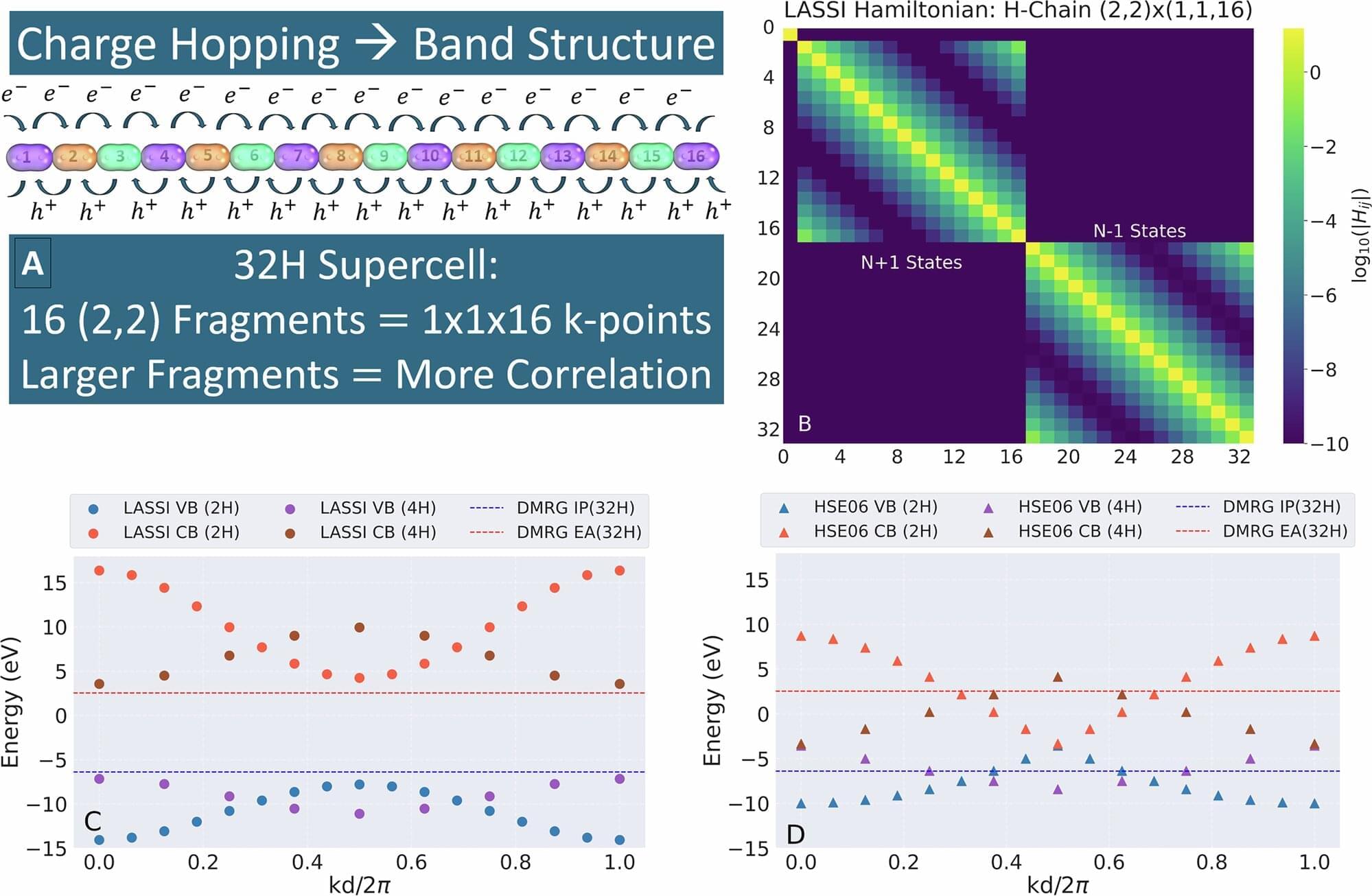
A study of some of the first net-zero-ready homes in the UK has found that their peak grid power demand is far lower than planners had anticipated. The research confirms that these all-electric homes can significantly cut energy use and emissions.
Buildings account for around 37% of global energy-related emissions, with residential properties making up approximately 17% of that total. In 2019, the UK government set an ambitious target to achieve net-zero greenhouse gas emissions by 2050. To help meet it, the Future Homes Standard requires all new homes built from 2025 to cut their carbon emissions by 75% to 80%.
Fully electric homes use technologies like air-source heat pumps (ASHPs) for heating (by extracting heat from outdoor air) and solar PV panels for electricity generation. But the big question has been whether they work as promised and achieve their energy efficiency goals in the real world.