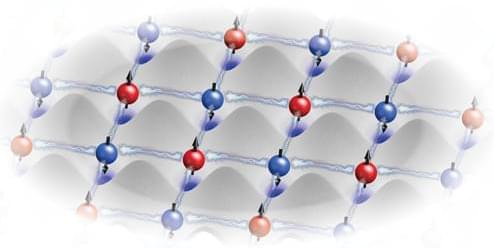
Quantum mechanically entangled groups of eight and ten ultracold atoms provide a critical demonstration for optical-lattice-based quantum processing.

Introducing the four fundamental forces of nature that describe (nearly) everything going on in our universe. This clip is taken from the Shots In The Quark T…
Trinity’s quantum physicists in collaboration with IBM Dublin have successfully simulated super diffusion in a system of interacting quantum particles on a quantum computer.
This is the first step in doing highly challenging quantum transport calculations on quantum hardware and, as the hardware improves over time, such work promises to shed new light in condensed matter physics and materials science.
The work is one of the first outputs of the TCD-IBM predoctoral scholarship programwhich was recently established where IBM hires Ph.D. students as employees while being co-supervised at Trinity. The paper was published recently in npj Quantum Information.
Researchers from the Department of Physics at Universität Hamburg, observed a quantum state that was theoretically predicted more than 50 years ago by Japanese theoreticians but so far eluded detection. By tailoring an artificial atom on the surface of a superconductor, the researchers succeeded in pairing the electrons of the so-called quantum dot, thereby inducing the smallest possible version of a superconductor. The work appears in the journal Nature.
Usually, electrons repel each other due to their negative charge. This phenomenon has a huge impact on many materials properties such as the electrical resistance. The situation changes drastically if the electrons are “glued” together to pairs thereby becoming bosons. Bosonic pairs do not avoid each other like single electrons, but many of them can reside at the very same location or do the very same motion.
One of the most intriguing properties of a material with such electron pairs is superconductivity, the possibility to let an electrical current flow through the material without any electrical resistance. For many years, superconductivity has found many important technological applications, including magnetic resonance imaging or highly sensitive detectors for magnetic fields.

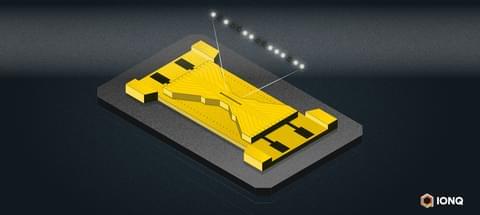
Not many pure-play quantum computing start-ups have dared to go public. So far, the financial markets have tended to treat the newcomers unsparingly. One exception is IonQ, who along with D-Wave and Rigetti, reported quarterly earnings last week. Buoyed by hitting key technical and financial goals, IonQ’s stock is up ~400% (year-to-date) and CEO Peter Chapman is taking an aggressive stance in the frothy quantum computing landscape where error correction – not qubit count – has increasingly taken center stage as the key challenge.
This is all occurring at a time when a wide variety of different qubit types are vying for dominance. IBM, Google, and Rigetti are betting on superconducting-based qubits. IonQ and Quantinuuum use trapped ions. Atom Computing and QuEra use neutral atoms. PsiQuantum and Xanadu rely on photonics-based qubits. Microsoft is exploring topological qubits based on the rare Marjorana particle. And more are in the works.
It’s not that the race to scale up qubit-count has ended. IBM has a 433-plus qubit device (Osprey) now and is scheduled to introduce 1100-qubit device (Condor) late this year. Several other quantum computer companies have devices in the 50–100 qubit range. IonQ’s latest QPU, Forte, has 32 qubits. The challenge they all face is that current error rates remain so high that it’s impractical to reliably run most applications on the current crop of QPUs.
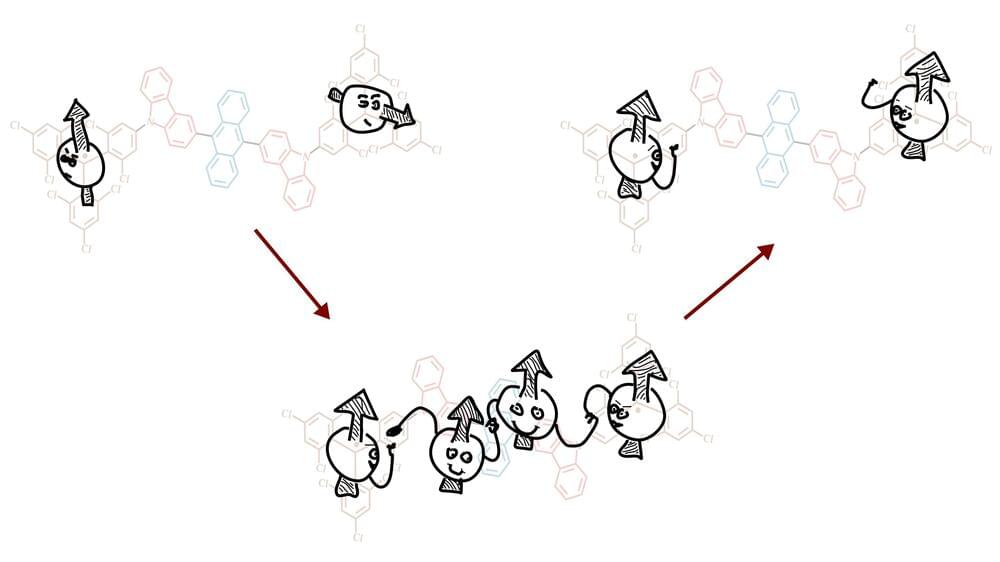
Researchers have found a way to control the interaction of light and quantum ‘spin’ in organic semiconductors, that works even at room temperature.
Spin is the term for the intrinsic angular momentum of electrons, which is referred to as up or down. Using the up/down spin states of electrons instead of the 0 and 1 in conventional computer logic could transform the way in which computers process information. And sensors based on quantum principles could vastly improve our abilities to measure and study the world around us.
An international team of researchers, led by the University of Cambridge, has found a way to use particles of light as a ‘switch’ that can connect and control the spin of electrons, making them behave like tiny magnets that could be used for quantum applications.
A mysterious quantum phenomenon reveals an image of an atom like never before. You can even see the difference between protons and neutrons.
The Relativistic Heavy Ion Accelerator (RHIC), from the Brookhaven Laboratory in the United States, is a sophisticated device capable of accelerating gold ions to a speed of up to 99.995% that of light. Thanks to him, it has recently been possible to verify, for example, Einstein’s famous equation E=mc2.

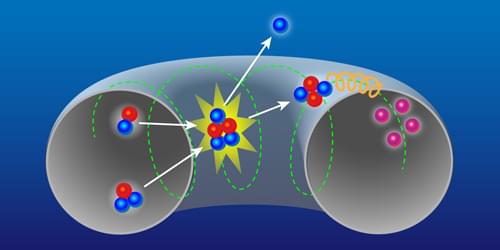
The observation of self-heating in magnetically confined plasmas represents a milestone on the road to fusion reactors based on such plasmas.
A fusion reactor would generate electricity using the energy released by nuclear-fusion reactions occurring in a plasma. A key step in the race toward realizing the dream of such a reactor is the creation of a burning plasma—one in which the fusion reactions themselves supply most of the heating needed to keep the plasma at fusion-relevant temperatures. This step has recently been demonstrated for inertially confined plasmas [1, 2] (see Research News: Ignition First in a Fusion Reaction) but has so far remained elusive for magnetically confined ones. This goal could now be within reach thanks to direct evidence for fusion-induced heating of electrons in magnetically confined plasmas obtained by Vasily Kiptily and colleagues at the UK-based Joint European Torus (JET) facility [3].
The fusion of two heavy hydrogen isotopes—deuterium (D) and tritium (T)—presents the most promising path to a fusion reactor, both because of the relative ease in getting these isotopes to fuse and because of the large amount of energy released in each reaction. When D and T fuse, an alpha particle (a helium-4 nucleus) and a neutron are generated, carrying the released energy in the form of kinetic energy. The goal of achieving energy production from controlled fusion on Earth relies on the created alpha particles remaining in the plasma and heating the fusion fuel to keep the reactions going, while the kinetic energy of neutrons escaping the plasma is converted to electrical energy.