Autoimmune disease occurs from the body’s immune system attacking its healthy cells. Unfortunately, the mechanism that would normally prevent autoimmunity is not present in some individuals. T cells are the immune cell population responsible for killing or lysing invading pathogens. In the context of autoimmunity, T cells attack and lyse healthy cells. The thymus gland educates or prepares T cells to become activated and target foreign pathogens. T cells are exposed to different molecules and surface markers which further train these cells on how to respond when they come into contact with foreign markers. Autoimmune disorders are rare and can often be detected in children. However, there are limited treatment options, and a cure has not been found. Researchers are currently working to better treat autoimmune disorders and improve the quality of life in patients.
A recent article published in Nature, by a team led by Dr. Thomas Korn, reported a previously unknown mechanism underlying autoimmune disease. Korn is a Professor of Experimental Nueroimmunology at the Technical University of Munich (TUM) and Principal Investigator at the Maximilian University of Munich (LMU). His lab focuses on T cell biology and the underlying mechanisms of autoimmune disorders. Korn and others demonstrated that another immune cell population, B cells, aid in T cell education in the thymus gland. Korn and others point out that B cells are part of T cell development and play a critical role in autoimmune disorder.
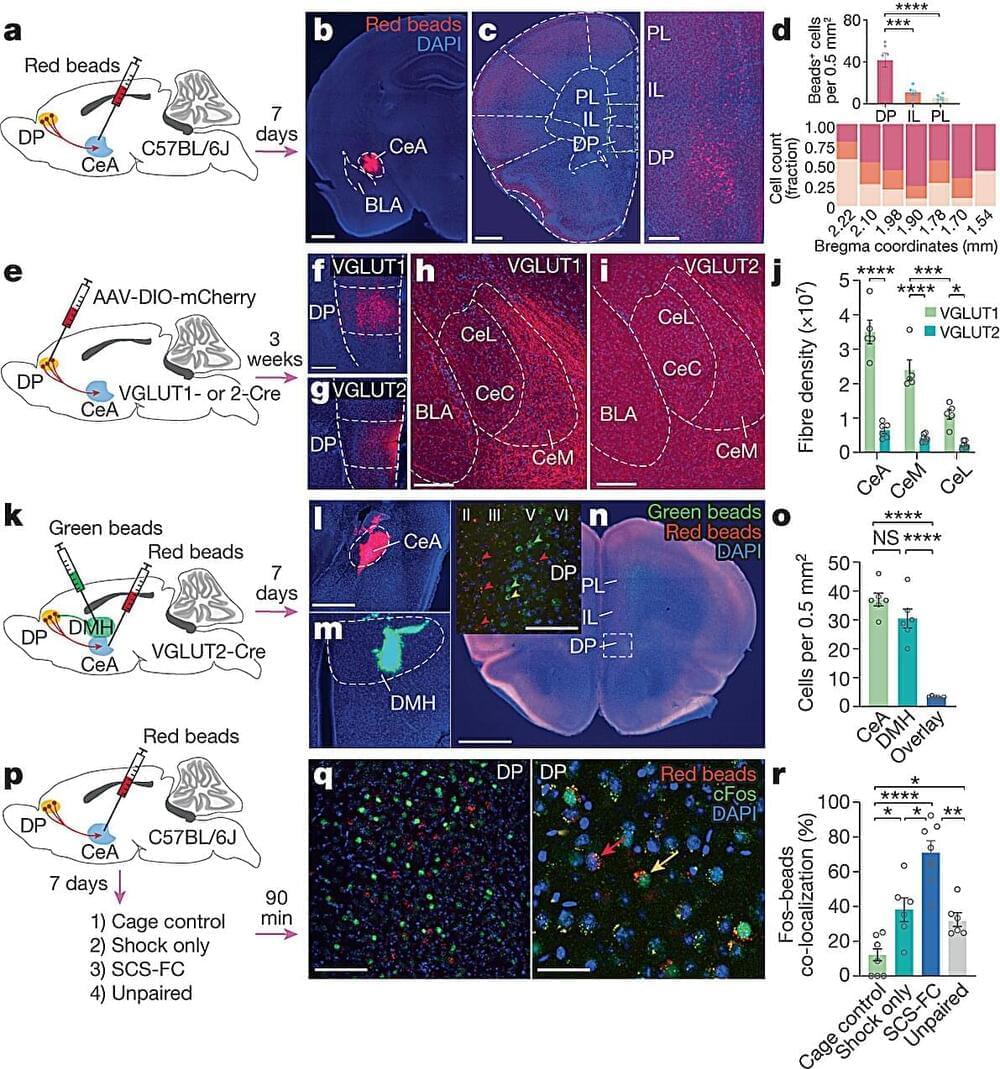
Researchers used both animal models and human tissue samples to conduct their research to investigate T cell development. The autoimmune disorder Korn and his team used as a model is known as neuromyelitis optica, which is similar to multiple sclerosis (MS). Researchers chose this specific model due to the well-known fact that T cells respond to the protein AQP4 in this autoimmune disorder. Interestingly, AQP4 is highly expressed in the nervous system, which becomes the target of autoimmunity. Researchers discovered that B cells also express AQP4, which present this protein to the T cells in the thymus. Interestingly, if the B cells did not express AQP4, then T cells would not become reactive to the surface protein and target healthy nervous system cells. Epithelial cells also expressed the AQP4 protein and resulted in the same autoimmune reaction. However, B cells were found to significantly impact T cell development compared to other cells in the thymus.