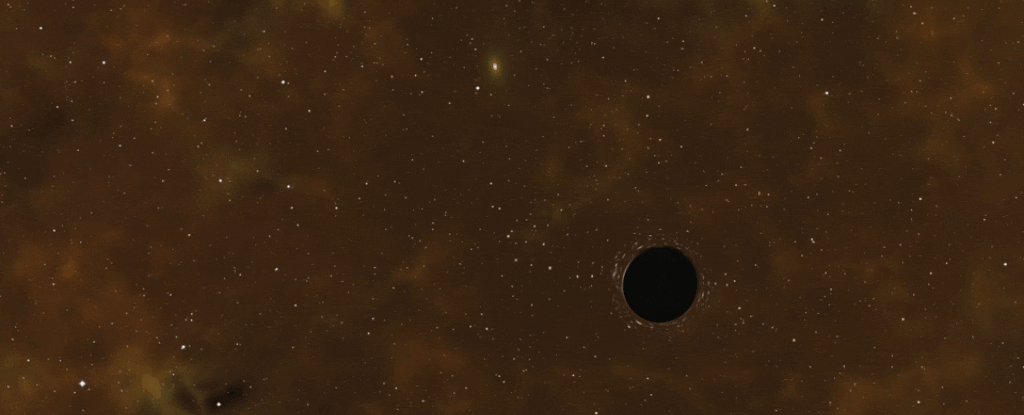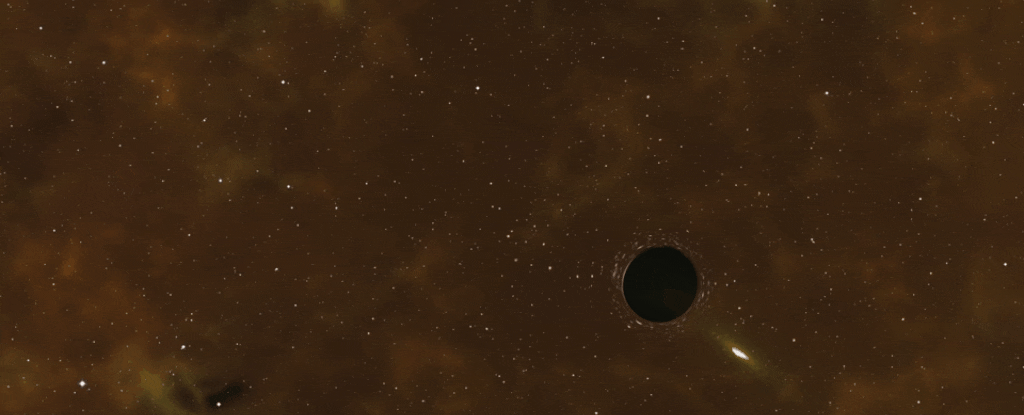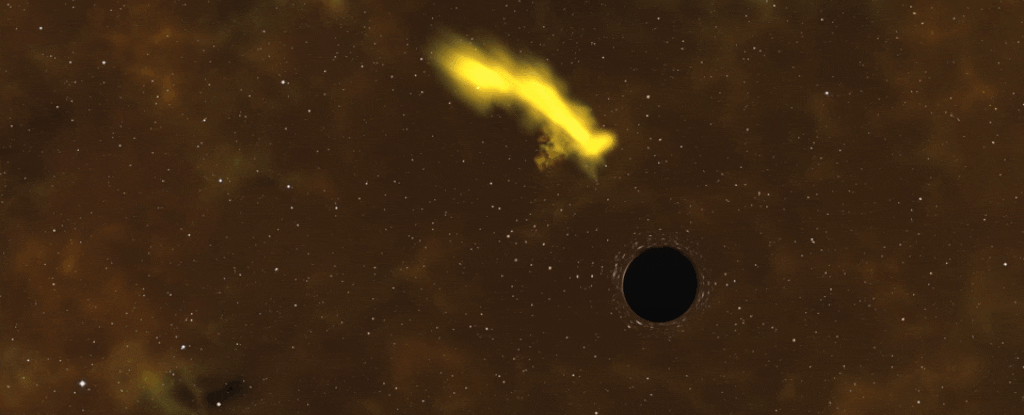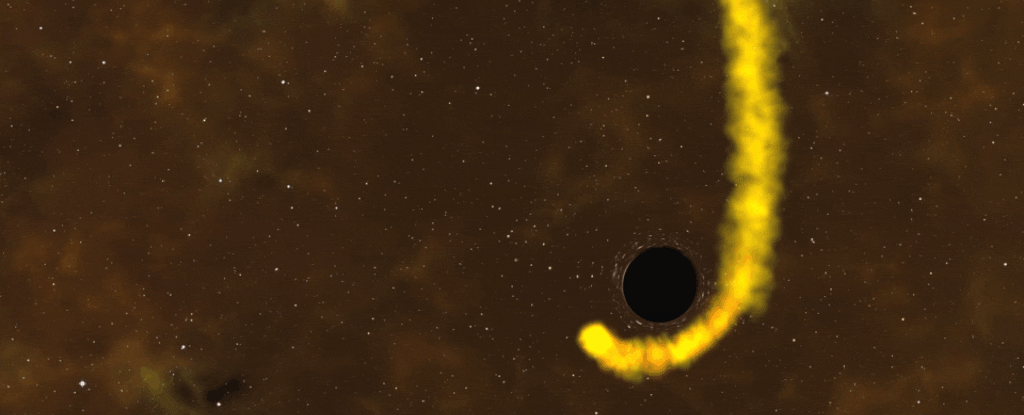
I don’t think that star is the same after that one night stand.
When black holes swallow down massive amounts of matter from the space around them, they’re not exactly subtle about it. They belch out tremendous flares of X-rays, generated by the material heating to intense temperatures as it’s sucked towards the black hole, so bright we can detect them from Earth.
This is normal black hole behaviour. What isn’t normal is for those X-ray flares to spew forth with clockwork regularity, a puzzling behaviour reported in 2019 from a supermassive black hole at the centre of a galaxy 250 million light-years away. Every nine hours, boom — X-ray flare.
After careful study, astronomer Andrew King of the University of Leicester in the UK identified a potential cause — a dead star that’s endured its brush with a black hole, trapped on a nine-hour, elliptical orbit around it. Every close pass, or periastron, the black hole slurps up more of the star’s material.