A trio of mRNA molecules could help guard against the harmful effects of aging on immune cells, a study in mice finds.


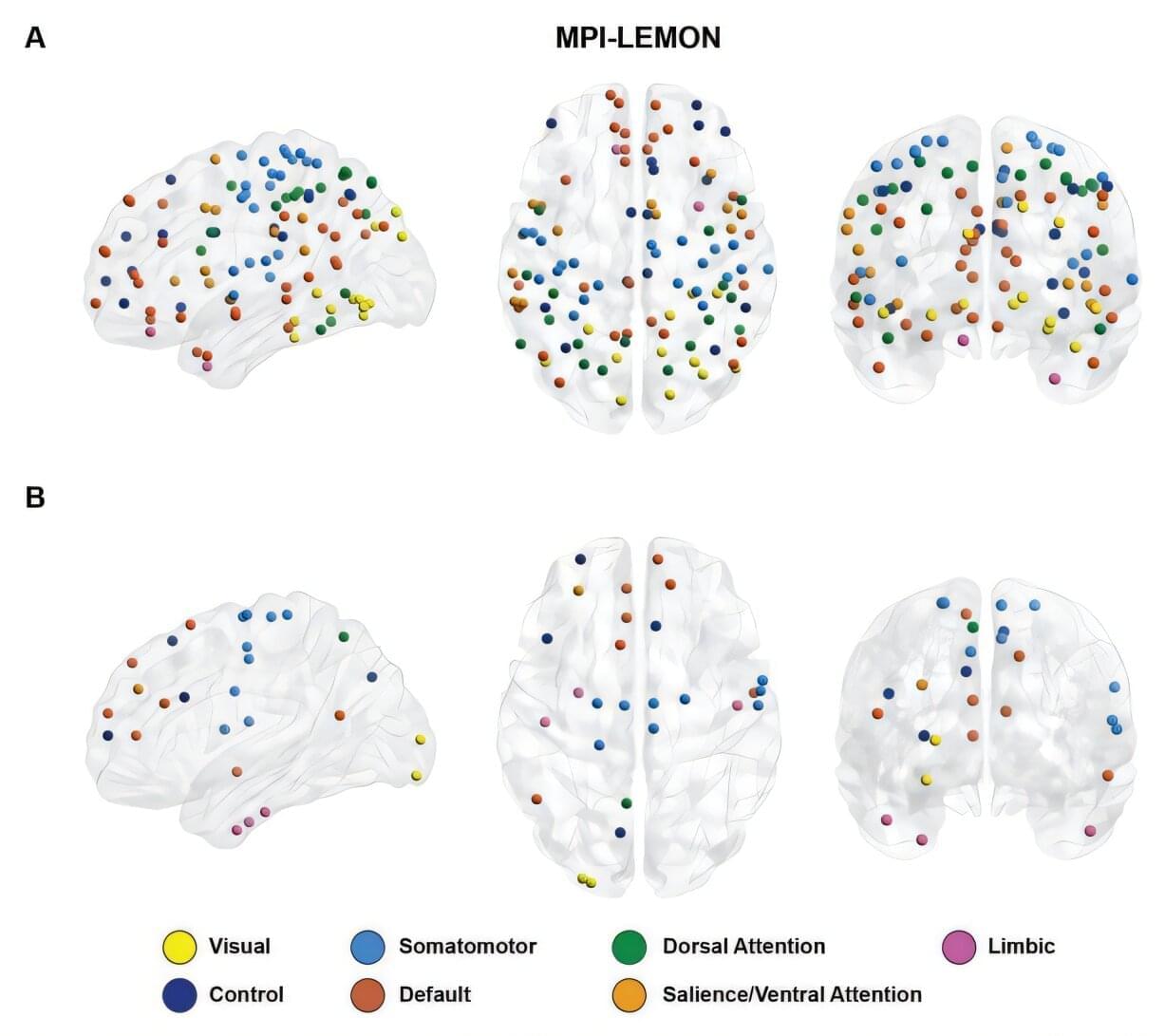
It is a central question in neuroscience to understand how different regions of the brain interact, how strongly they “talk” to each other. Researchers from the Max Planck Institute for Mathematics in the Sciences Leipzig, Germany, the Institute of Mathematical Sciences in Chennai, India, and colleagues demonstrate how mathematical techniques from topological data analysis (TDA) can provide a new, multiscale perspective on brain connectivity. The study was published in the journal Patterns.
With the rise of large neuroimaging datasets, scientists now work with detailed maps of brain connectivity—network representations that show how hundreds of brain regions fluctuate and coordinate their activity over time. But making sense of these enormous networks poses a challenge: What patterns matter? Which changes signal healthy aging, and which reflect differences associated with autism spectrum disorder (ASD)?
The study introduces a mathematical innovation that helps answer precisely these questions. Researchers applied persistent homology, a tool from topological data analysis (TDA), to detect how brain connectivity reorganizes during healthy aging and in ASD.
Questions to inspire discussion.
🤖 Q: How quickly will AI and robotics replace human jobs? A: AI and robotics will do half or more of all jobs within the next 3–7 years, with white-collar work being replaced first, followed by blue-collar labor through humanoid robots.
🏢 Q: What competitive advantage will AI-native companies have? A: Companies that are entirely AI-powered will demolish competitors, similar to how a single manually calculated cell in a spreadsheet makes it unable to compete with entirely computer-based spreadsheets.
💼 Q: What forces companies to adopt more AI? A: Companies using more AI must outcompete those using less, creating a forcing function for increased AI adoption, as inertia currently keeps humans doing AI-capable tasks.
📊 Q: How much of enterprise software development can AI handle autonomously? A: Blitzy, an AI platform using thousands of specialized agents, autonomously handles 80%+ of enterprise software development, increasing engineering velocity 5x when paired with human developers.
Energy and Infrastructure.
The first NMNH human trial shows NAD+ levels increased up to 3x in 90 days. Here’s what the data actually reveal—and what’s still missing.
Some links are affiliate links so we will earn a commission when they are used to purchase products.
If you would like to support our channel please consider joining our patreon / modernhealthspan.
~~~~~~~~~~~~~~
ProHealth (15% off: MODERN) https://prohealth.pxf.io/aObQRR NMNH 500mg.
Renue By Science (10% off: MHS) https://tinyurl.com/bdew4bfs NMN Powder • Lipo NR
BiOptimizers (15% off: MHBIO) https://bit.ly/47VAa8f — Magnesium Breakthrough.
Stemregen (15% off: MODERN) US only https://tinyurl.com/45z968yr.
Oxford Healthspan (15% off: MHS) https://tinyurl.com/hrxfnzpn — Spermidine.
Seeking Health (10% off: Richard10) https://crrnt.app/SEEK/-dm0MyrQ, Histamine Nutrients https://crrnt.app/SEEK/EpM7paAO
Qualia (15% off: MODERN) https://bit.ly/4jlaibe, Senolytic.
Wellness Extract (10% off: MODERNWE) http://wellnessextract.com/RICHARDWE Geranylgeraniol • Vit E
AX3 Life (20% off: MODERN20) https://tinyurl.com/2t3w26nw — Astaxanthin.
~~~~~~~~~~~~~~
The first human clinical trial results for NMNH are here. In this 90-day randomized, double-blind, placebo-controlled study, NMNH increased NAD+ levels up to 3x in healthy adults, with participants reporting improvements in energy and fatigue. But before you get too excited, there are important limitations to understand.
In this video, I break down the trial design, explain what the NAD+ increases actually mean, review the subjective outcomes like energy and emotional well-being (measured via SF-36), and discuss why the biological age claims are difficult to interpret. I also cover safety data and what we still need to know.
This is promising early data for NMNH vs NMN, but it’s unpublished, lacks key details, and needs independent replication. Here’s everything you need to know about what this trial does and doesn’t tell us.
Key topics: NMNH clinical trial, NAD+ boosters, NMN vs NMNH, longevity supplements, anti-aging research, NAD+ levels, UthPeak study, Phase I trial results.
📚 Chapters.
0:00 — Introduction & Trial Overview What this video covers and the trial basics.
1:39 — Trial Design & Methodology Study structure, participants, and objectives.
2:31 — NAD+ Results The primary outcome: dose-dependent increases.
3:18 — Subjective Outcomes & Limitations Energy, mood, biological age claims, and why interpretation is difficult.
6:26 — Safety & Final Thoughts Tolerability data and what comes next.
🌐Links in this video.
NMNH Clinical Trial https://www.clinicaltrials.gov/study/.… nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice https://faseb.onlinelibrary.wiley.com… Reduced Nicotinamide Mononucleotide (NMNH) Potently Enhances NAD+ and Suppresses Glycolysis, the TCA Cycle, and Cell Growth https://pubmed.ncbi.nlm.nih.gov/33793… *************************************** Health claims Disclosure: Information provided on this video is not a substitute for direct, individual medical treatment or advice. Please consult with your doctor first. Products or services mentioned in this video are not a recommendation. Audio Copyright Disclaimer Please note that we have full authorization to the music that we used in our videos as they were created using the service WeVideo which provides the rights to the music. The rights are detailed in the terms of use that can be reviewed here https://www.wevideo.com/terms-of-use and any following inquiries should be addressed to [email protected]. ********************************************** #nmnh #nad #nmn.
Reduced nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice.
https://faseb.onlinelibrary.wiley.com…
Reduced Nicotinamide Mononucleotide (NMNH) Potently Enhances NAD+ and Suppresses Glycolysis, the TCA Cycle, and Cell Growth.
https://pubmed.ncbi.nlm.nih.gov/33793…
*******************************
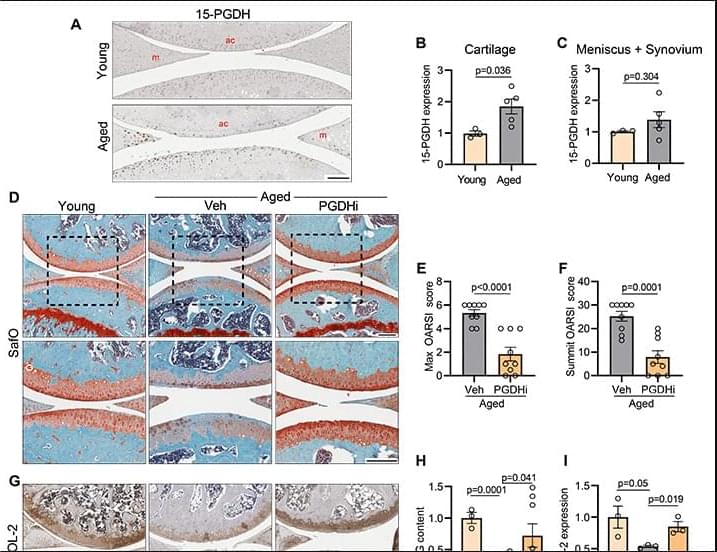
Aging or injury to the joints can lead to cartilage degeneration and osteoarthritis (OA), for which there are limited effective treatments. We found that expression of 15-hydroxy prostaglandin dehydrogenase (15-PGDH) is increased in the articular cartilage of aged or injured mice. Both systemic and local inhibition of 15-PGDH with a small molecule inhibitor (PGDHi) led to regeneration of articular cartilage and reduction in OA-associated pain. Using single cell RNA-sequencing and multiplexed immunofluorescence imaging of cartilage, we identified the major chondrocyte subpopulations. Inhibition of 15-PGDH decreased hypertrophic-like chondrocytes expressing 15-PGDH and increased extracellular matrix-synthesizing articular chondrocytes. Cartilage regeneration appears to occur through gene expression changes in pre-existing chondrocytes, rather than stem or progenitor cell proliferation.
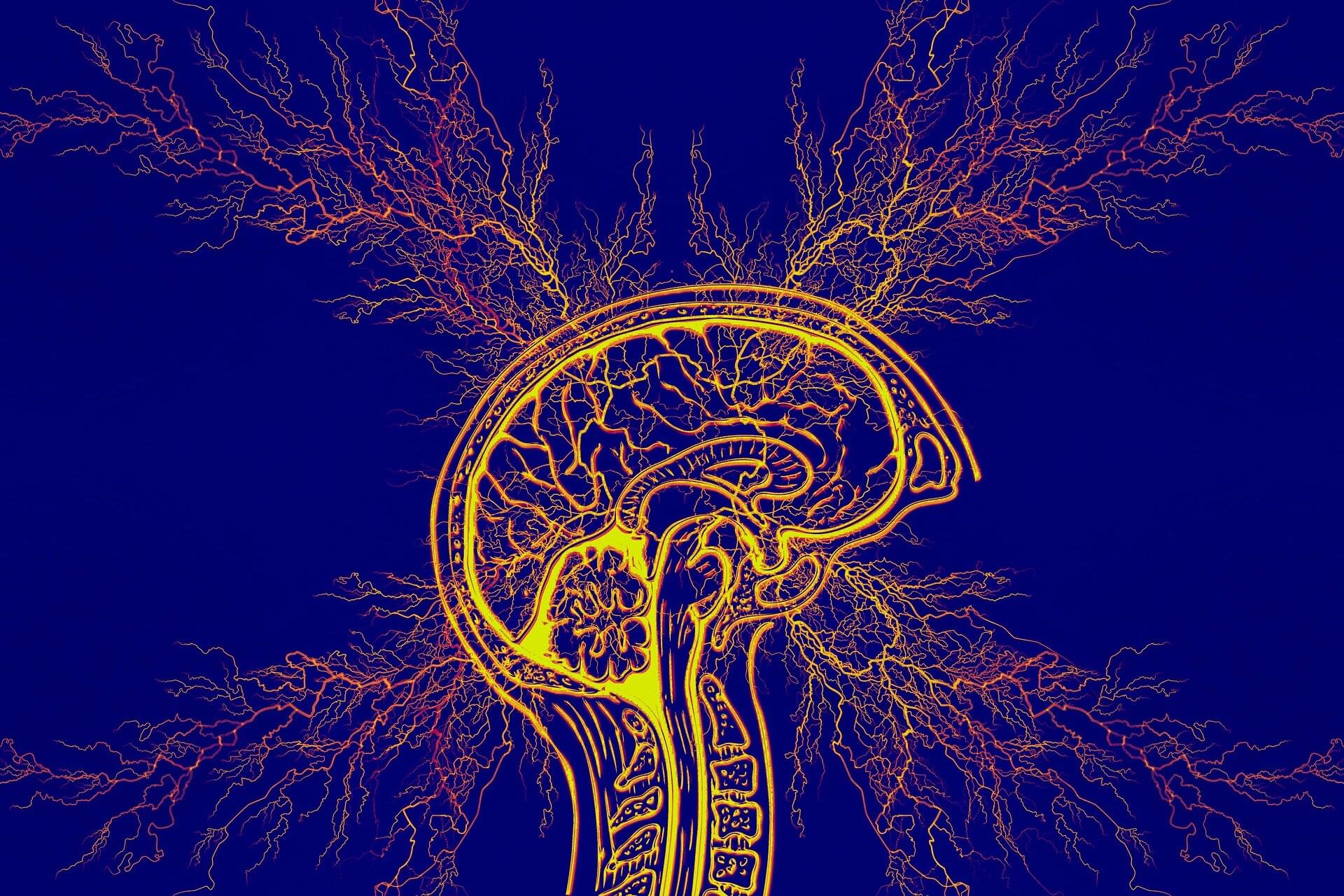
Temporal lobe epilepsy, which results in recurring seizures and cognitive dysfunction, is associated with premature aging of brain cells.
A new study by researchers at Georgetown University Medical Center found that this form of epilepsy can be treated in mice by either genetically or pharmaceutically eradicating the aging cells, thereby improving memory and reducing seizures as well as protecting some animals from developing epilepsy.
The study appears in the journal Annals of Neurology.

We present the first comprehensive data set for the aluminium content of brain tissue in donors without a diagnosis of neurodegenerative disease. All donors fulfilled recently revised criteria for control brain tissues14. Approximately 80% of measured tissues have an aluminium content below 1.0 μg/g dry wt. (Table 1). There are some anomalies, 6 out of 191 tissues have an aluminium content ≥3.00 μg/g dry wt., and these are worth future investigation to identify possible neuropathology. There was no statistically significant relationship between brain aluminium content and age of donor and this observation is contrary to a previous investigation of brain aluminium in a neurologically normal population15. An explanation may be that herein only two out of twenty donors were below 66 years old. The data do support a conclusion that a high content of brain aluminium is not an inevitability of ageing.
When we compared the new control data set with data produced in an identical manner in donors dying with diagnoses of sporadic Alzheimer’s disease (sAD)16, familial Alzheimer’s disease (fAD)11, autism spectrum disorder (ASD)13 and multiple sclerosis (MS)12 all of these disease groups had significantly higher brain aluminium content. The differences were always highly significant regardless of the method of statistical analysis (Table 4). The largest disease group, designated as sAD, was actually composed of approximately equal numbers of donors previously described by a brain bank as controls and donors diagnosed with sAD. Unfortunately, information discriminating between control and sAD donors was not made available to us17. However, the observation that the aluminium content of brain tissue in this group as a whole was significantly higher than the similarly aged control group emphasised the likelihood that brain aluminium content is increased in sAD.
Can humans live for thousands of years? New DNA and longevity research suggests that aging may not be fixed—it may simply be the result of imperfect cellular repair. In this video, we explore how DNA damage, genetic repair mechanisms, and modern longevity science are reshaping our understanding of human lifespan.
This content is based on current research from USA and Europe, focusing on emerging breakthroughs in genetics, DNA repair therapies, and anti-aging science.
If you’re interested in health, biology, or the future of human longevity, this video is for you.
Disclaimer:
This video is for educational purposes only, is not intended to diagnose, treat, or cure any condition, and does not replace professional medical advice. Always consult a qualified healthcare provider for guidance related to your health.
#LongevityScience.
#DNARepair.
#AntiAging.
#GeneticsResearch.
#HealthFacts.
#BioLogicHealth.
#ScienceExplained.
#HealthyAging