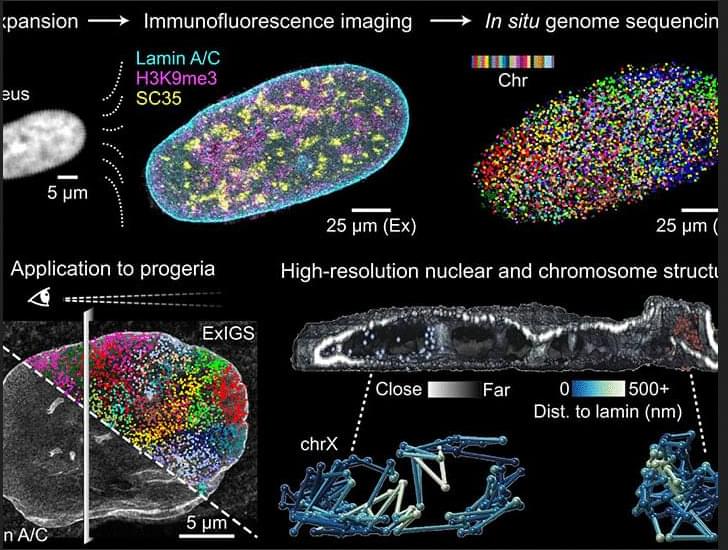
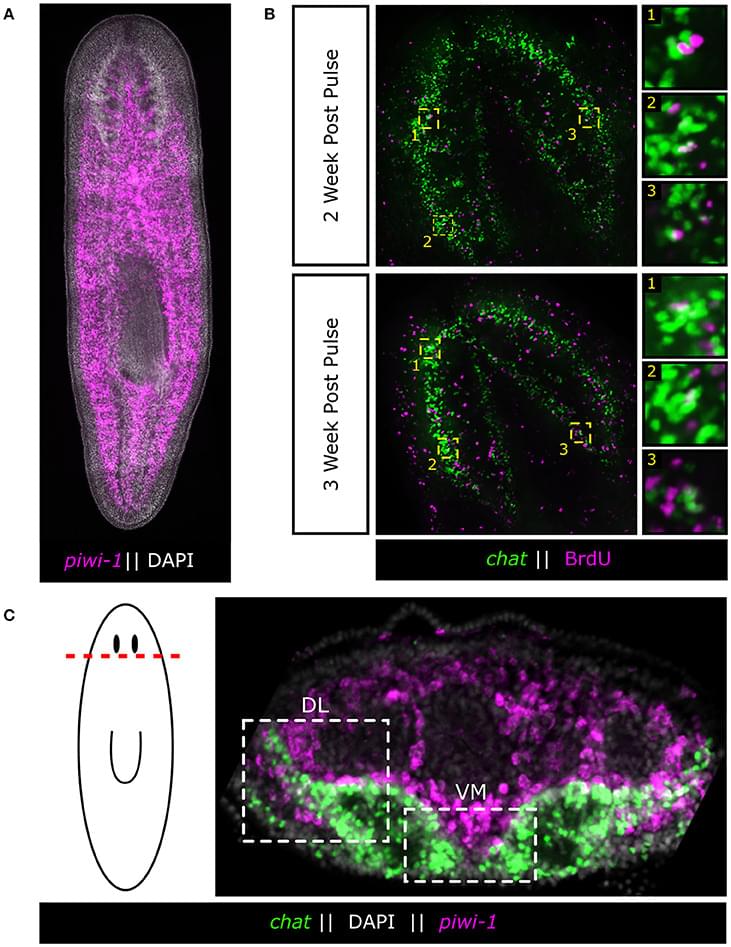
The human cerebral cortex is only a few millimetres thick and arranged in numerous folds. This tissue usually becomes thinner with age. “This is a hallmark of aging. It is attributed, among other things, to the loss of neurons. As a result, some abilities deteriorate. In any case, it is generally assumed that less brain volume means reduced function,” explains Prof. Esther Kühn, a neuroscientist at DZNE and the Hertie Institute for Clinical Brain Research. “However, little is known about how exactly the cortex actually ages. This is remarkable, given that many of our daily activities depend on a functioning cortex. That’s why we examined the situation with high-resolution brain scans.”
Together with colleagues from Tübingen and Magdeburg, Esther Kühn focused on a part of the cerebral cortex where signals from the tactile sense are processed. This “primary somatosensory cortex” is located on the left and right side of the top of the head and extends along a strip about a finger’s width wide towards each ear. “This brain area is relevant for the perception of one’s own body and for interacting with the environment,” explains the neuroscientist. “When I pick up a key, grasp a door handle or even walk, I constantly need haptic feedback to control my movements. The corresponding stimuli converge in this area and are also processed here”
Using magnetic resonance imaging (MRI), the researchers were able to map this area of the cerebral cortex with unprecedented accuracy. To do this, they employed a particularly sensitive scanner with a magnetic field strength of seven Tesla, enabling them to image minute brain structures about the size of a grain of sand. A total of around 60 women and men between the ages of 21 and 80 were examined. “Until now, it had not been considered that the primary somatosensory cortex consists of a stack of several extremely thin layers of tissue, each with its own architecture and function. We have now found that these layers age differently. Although the cerebral cortex becomes thinner overall, some of its layers remain stable or, surprisingly, are even thicker with age. Presumably because they are particularly solicited and thus retain their functionality. We therefore see evidence for neuroplasticity, that is, adaptability, even in senior people.”