The oil kingdom fears that its population is aging at an accelerated rate and hopes to test drugs to reverse the problem. First up might be the diabetes drug metformin.


Circa 2021 Immortality of the male genitalia in humans.
Cavernous nerve injury (CNI) is the main cause of erectile dysfunction (ED) following pelvic surgery. Our previous studies have demonstrated that transplantation of different sources of mesenchymal stem cells (MSCs) was able to alleviate ED induced by CNI in rat models. However, little is known about the therapeutic effects of human gingiva-derived MSCs (hGMSCs) in CNI ED rats. Herein, we injected the hGMSCs around the bilateral major pelvic ganglia (MPG) in a rat model of CNI and evaluated their efficacy. The results showed that treatment of hGMSCs could significantly promote the recovery of erectile function, enhance smooth muscle and endothelial content, restore neuronal nitric oxide synthase (nNOS) expression, and attenuate cell apoptosis in penile tissue. Moreover, penile fibrosis was significantly alleviated after hGMSC administration. In addition, potential mechanism exploration indicated that hGMSCs might exert its functions via skewed macrophage polarity from M1 toward M2 anti-inflammatory phenotype. In conclusion, this study found that transplantation of hGMSCs significantly improved CNI-related ED, which might provide new clues to evaluate their pre-clinical application.
There are many causes of erectile dysfunction (ED), which include psychological factors, neurological disorders (such as multiple sclerosis, temporal lobe epilepsy, and cavernous nerve injury), and vasculogenic disorders (such as atherosclerosis, hypertension, and diabetes mellitus). Neurogenic sexual dysfunction makes up about 10–19% in all causes of erectile dysfunction. Neurotic erectile dysfunction is one of most important complications after radical prostatectomy and rectectomy, owing to intraoperative damage of the pelvic cavernous nerve (CN). It affects not only the physical but also mental health in postoperative patients. Despite the improvement of nerve-sparing techniques, the incidence of neurotic ED still has no substantial improvement. The incidences of ED range from 75 to 80% after pelvic surgery (Schauer et al., 2015).

Circa 2021 Secretomes of human pluripotent stem cell-derived smooth muscle cell progenitors upregulate extracellular matrix metabolism in the lower urinary tract and vagina.
Adult mesenchymal stem cells (MSCs) have been studied extensively for regenerative medicine; however, they have limited proliferation in vitro, and the long culture time induces cell senescence. MSCs also contribute to tissue repair through their paracrine function. In this study, we sought to examine the paracrine effects of human smooth muscle cell progenitors (pSMC) on the urethra and adjacent vagina of stress urinary incontinence rodents. We use human pluripotent stem cell (PSC) lines to derive pSMCs to overcome the issue of decreased proliferation in tissue culture and to obtain a homogenous cell population.
Three human PSC lines were differentiated into pSMCs. The conditioned medium (CM) from pSMC culture, which contain pSMC secretomes, was harvested. To examine the effect of the CM on the extracellular matrix of the lower urinary tract, human bladder smooth muscle cells (bSMCs) and vaginal fibroblasts were treated with pSMC-CM in vitro. Stress urinary incontinence (SUI) was induced in rats by surgical injury of the urethra and adjacent vagina. SUI rats were treated with pSMC-CM and monitored for 5 weeks. Urethral pressure testing was performed prior to euthanasia, and tissues were harvested for PCR, Western blot, and histological staining. Kruskal-Wallis one-way ANOVA test and Student t test were used for statistical comparisons.
pSMC-CM upregulated MMP-2, TIMP-2, collagen, and elastin gene expression, and MMP-9 activity in the human bladder and vaginal cells consistent with elastin metabolism modulation. pSMC-CM treatment in the SUI rat improved urethral pressure (increase in leak point pressure compared to intact controls, p 0.05) and increased collagen and elastin expression in the urethra and the adjacent vagina.

Could drinking coffee lead to a longer lifespan?
Many people will swear to the life extending properties of coffee, be it saving them from keeling over from exhausting in the early hours of the morning or saving an annoying co-worker from the unbridled rage of someone who hasn’t yet acquired their caffeine fix. Yes, coffee is without a doubt one of the most powerful (and mostly metaphorical) lifesavers of the modern world. However, recent studies into the effects of drinking coffee on human lifespan have found that it might very well have a significant impact on health and longevity. A study of 170,000 people from the UK found that those who drank between two and four cups of coffee a day were 30% less likely to die from all causes compared to those who did not drink coffee at all. Interestingly, the same study also revealed that taking a small amount of sugar with their coffee had no significant detrimental effects on the health of the coffee drinker. This is not the first study that has made such claims about the health benefits of coffee, in 2019 a team of scientists showed that on average coffee drinkers could be expected to live on average 2 years longer than those who did not consume the beverage.
So why coffee? What is it about this common beverage that is having such a significant impact on the health of those who drink it? The immediate conclusion one might be drawn to is that is has something to do with the caffeine found within the coffee, as this is commonly regarded as the most significant feature (or indeed to some, the entire point) of coffee. It certainly makes us feel more alert, increases our heart rate, and even increases our metabolism. However, this might very well not have anything to do with how coffee is actually helping to extend life. A similar study conducted by Harvard involving 500,000 British coffee drinkers (which linked coffee to a 14% lower risk of death) found that both caffeinated and decaffeinated coffee both improved an individual’s longevity, indicated that caffeine may not be what is responsible for the increased lifespan enjoyed by coffee drinkers.
Scientists successfully proved reverse aging techniques in mice by employing proteins that can turn an adult cell into a stem cell. There’s hope for you yet.
Circa 2019 immortality in the human brain 🧠
Brain injuries causing chronic sensory or motor deficit, such as stroke, are among the leading causes of disability worldwide, according to the World Health Organization; furthermore, they carry heavy social and economic burdens due to decreased quality of life and need of assistance. Given the limited effectiveness of rehabilitation, novel therapeutic strategies are required to enhance functional recovery. Since cell-based approaches have emerged as an intriguing and promising strategy to promote brain repair, many efforts have been made to study the functional integration of neurons derived from pluripotent stem cells (PSCs), or fetal neurons, after grafting into the damaged host tissue. PSCs hold great promises for their clinical applications, such as cellular replacement of damaged neural tissues with autologous neurons. They also offer the possibility to create in vitro models to assess the efficacy of drugs and therapies. Notwithstanding these potential applications, PSC-derived transplanted neurons have to match the precise sub-type, positional and functional identity of the lesioned neural tissue. Thus, the requirement of highly specific and efficient differentiation protocols of PSCs in neurons with appropriate neural identity constitutes the main challenge limiting the clinical use of stem cells in the near future. In this Review, we discuss the recent advances in the derivation of telencephalic (cortical and hippocampal) neurons from PSCs, assessing specificity and efficiency of the differentiation protocols, with particular emphasis on the genetic and molecular characterization of PSC-derived neurons. Second, we address the remaining challenges for cellular replacement therapies in cortical brain injuries, focusing on electrophysiological properties, functional integration and therapeutic effects of the transplanted neurons.
Brain injuries represent a large variety of disabling pathologies. They may originate from different causes and affect distinct brain locations, leading to an enormous multiplicity of various symptoms ranging from cognitive deficits to sensorimotor disabilities. They can also result in secondary disturbances, such as epileptic foci, which occur within the lesioned and perilesional tissues (Herman, 2002). Indeed, frequently a secondary functional damage can take place in a region distant from the first insult (e.g., the hippocampus after traumatic brain injury), providing an explanation for cognitive and memory deficits arising after a brain lesion (Girgis et al., 2016). Brain injuries can have traumatic or non-traumatic etiologies, including focal brain lesions, anoxia, tumors, aneurysms, vascular malformations, encephalitis, meningitis and stroke (Teasell et al., 2007). In particular, stroke covers a vast majority of acquired brain lesions.

A team of scientists at a company called 3DBio Therapeutics have successfully transplanted a 3D printed ear made from the patient’s own cells, The New York Times reports.
It appears to be a first in the field of tissue engineering, according to experts, and could be the harbinger of a new era of regenerative medicine.
“It’s definitely a big deal,” Carnegie Mellon biomedical engineering researcher Adam Feinberg, who was not involved in the project, told the NYT. “It shows this technology is not an ‘if’ anymore, but a ‘when.’”.

Supplementing your diet with the sea organisms Ascidiacea, also known as sea squirts, reverses some of the main signs of aging, according to a new study using an animal model.
While the Fountain of Youth, the mythical spring that restores youth to anyone who bathes in it or drinks its waters, is clearly fantasy, scientists are hard at work looking for ways to combat aging. Some of these scientists just had a breakthrough: they discovered that supplementing a diet with sea squirts, reverses some of the main signs of aging. While more research is needed to verify the effect in humans, as the study was conducted using mice, the findings are very promising.
If you’ve ever glanced in the mirror and seen greying hair and wrinkles, or if you’ve forgotten the name of a close friend, you may desire a medication that might halt or even reverse the effects of aging.
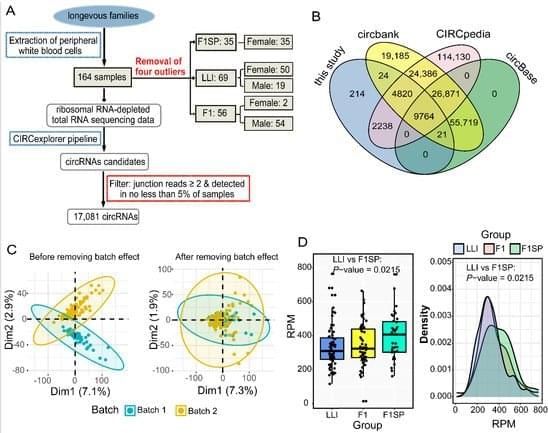
Deep RNA sequencing of 164 blood samples collected from long-lived families was performed to investigate the expression patterns of circular RNAs (circRNAs). Unlike that observed in previous studies, circRNA expression in long-lived elderly individuals (98.3 ± 3.4 year) did not exhibit an age-accumulating pattern. Based on weighted circRNA co-expression network analysis, we found that longevous elders specifically gained eight but lost seven conserved circRNA-circRNA co-expression modules (c-CCMs) compared with normal elder controls (spouses of offspring of long-lived individuals, age = 59.3 ± 5.8 year). Further analysis showed that these modules were associated with healthy aging-related pathways. These results together suggest an important role of circRNAs in regulating human lifespan extension.