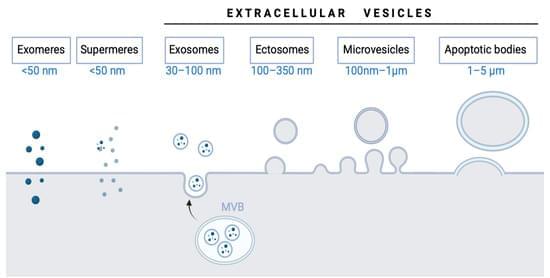
In recent decades, extracellular vesicles have been recognized as “very important particles” (VIPs) associated with aging and age-related disease. During the 1980s, researchers discovered that these vesicle particles released by cells were not debris but signaling molecules carrying cargoes that play key roles in physiological processes and physiopathological modulation. Following the International Society for Extracellular Vesicles (ISEV) recommendation, different vesicle particles (e.g., exosomes, microvesicles, oncosomes) have been named globally extracellular vesicles. These vesicles are essential to maintain body homeostasis owing to their essential and evolutionarily conserved role in cellular communication and interaction with different tissues. Furthermore, recent studies have shown the role of extracellular vesicles in aging and age-associated diseases.