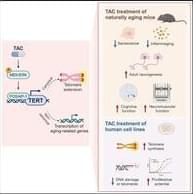
The enzyme telomerase can prevent telomere attrition from happening by extending the length of telomeres. However, in most multicellular organisms, including humans, telomerase expression is switched off, except in germ cells, some types of stem cells, and certain white blood cells. While this might play a role in preventing cancer, as most cancerous cells must switch telomerase expression back on via mutations to enable runaway replication, numerous studies have shown that increasing telomerase through TERT delays aging and increases longevity of model organisms [1].
The small molecule that could
In the lab, this is usually done by introducing genetic vectors carrying a working copy of the gene that codes TERT. It’s this gene that is switched off in somatic cells. However, gene therapies are complex and expensive, and they are just entering the medical mainstream. What if we could do the same using a small molecule?