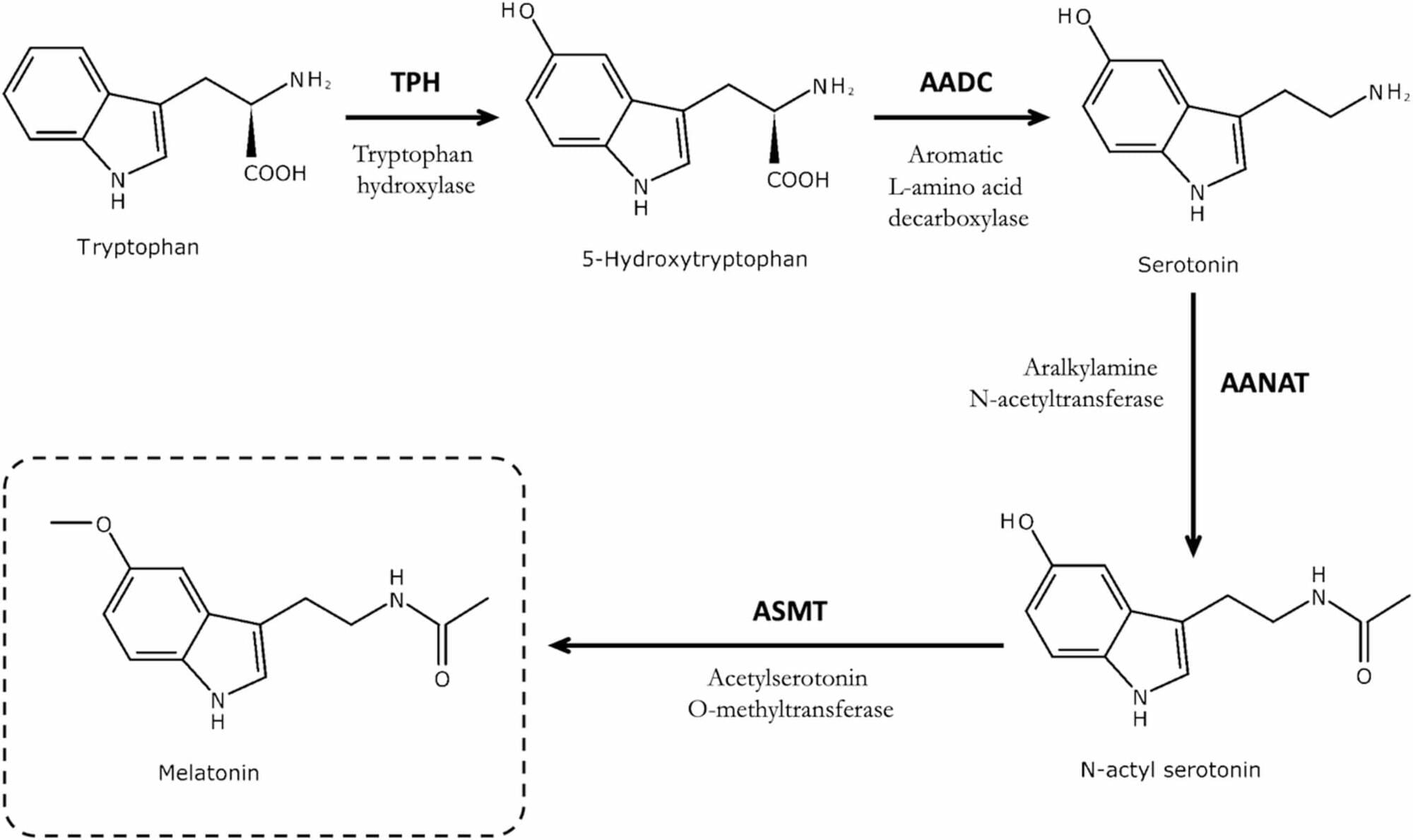
Epigenetic clocks, based on DNA methylation profiles at CpG sites, are widely recognized as reliable biomarkers of biological aging. However, common single-nucleotide polymorphisms (cSNPs), genomic variants that can overlap CpG sites, may affect DNA methylation profiles in ways that potentially interfere with the accuracy of epigenetic clocks. Moreover, because the prevalence of cSNPs varies across populations, such cSNP-CpG overlaps may differentially affect the age predictions of epigenetic clocks in diverse cohorts. Here, we present the first systematic cross-ancestry evaluation of cSNP robustness in the epigenetic clock, examining how cSNP-CpG overlaps affect the performance of epigenetic clocks across nine major genomic ancestry groups. We employed three complementary strategies: (a) testing whether cSNP-CpG overlaps are overrepresented in established epigenetic clocks or particular populations, (b) evaluating whether overlapping CpG sites correspond to the most influential aging predictors within clock models, and © simulating the effects of cSNP-associated methylation changes on predicted biological age. Our findings indicate that cSNP-CpG overlaps are not enriched among the CpG sites used in current epigenetic clocks, nor do they tend to involve the most influential sites. Furthermore, our simulation analysis revealed that current epigenetic clocks appear robust to cSNP-related methylation variations. Our findings underscore the overall stability of current epigenetic clocks, even in the presence of population-specific cSNP-CpG overlaps that are known to affect DNA methylation levels.
The authors have declared no competing interest.