Axolotls, hydra, and zebrafish can regenerate organs and bodies. Scientists say they may hold the key to healing, longevity, and scar-free recovery.


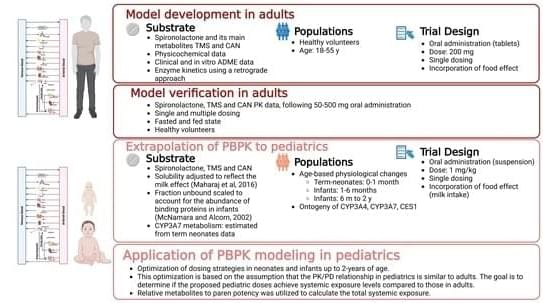
Background/Objectives: Spironolactone (SP) has been used off-label in pediatrics since its approval, but its use is challenged by limited pharmacokinetic (PK) data in adults and especially in children. Methods: Physiologically based pharmacokinetic (PBPK) models for SP and its active metabolites, canrenone (CAN) and 7α thio-methyl spironolactone (TMS), in adults were developed. These models aim to enhance understanding of SP’s PK and provide a basis for predicting PK and optimizing SP dosing in infants and neonates. Given SP’s complex metabolism, we assumed complete conversion to CAN and TMS by CES1 enzymes, fitting CES1-mediated metabolism to the parent-metabolite model using PK data. We incorporated ontogeny for CES1 and CYP3A4 and other age-related physiological changes into the model to anticipate PK in the pediatric population.
Epigenetic clocks, based on DNA methylation profiles at CpG sites, are widely recognized as reliable biomarkers of biological aging. However, common single-nucleotide polymorphisms (cSNPs), genomic variants that can overlap CpG sites, may affect DNA methylation profiles in ways that potentially interfere with the accuracy of epigenetic clocks. Moreover, because the prevalence of cSNPs varies across populations, such cSNP-CpG overlaps may differentially affect the age predictions of epigenetic clocks in diverse cohorts. Here, we present the first systematic cross-ancestry evaluation of cSNP robustness in the epigenetic clock, examining how cSNP-CpG overlaps affect the performance of epigenetic clocks across nine major genomic ancestry groups. We employed three complementary strategies: (a) testing whether cSNP-CpG overlaps are overrepresented in established epigenetic clocks or particular populations, (b) evaluating whether overlapping CpG sites correspond to the most influential aging predictors within clock models, and © simulating the effects of cSNP-associated methylation changes on predicted biological age. Our findings indicate that cSNP-CpG overlaps are not enriched among the CpG sites used in current epigenetic clocks, nor do they tend to involve the most influential sites. Furthermore, our simulation analysis revealed that current epigenetic clocks appear robust to cSNP-related methylation variations. Our findings underscore the overall stability of current epigenetic clocks, even in the presence of population-specific cSNP-CpG overlaps that are known to affect DNA methylation levels.
The authors have declared no competing interest.
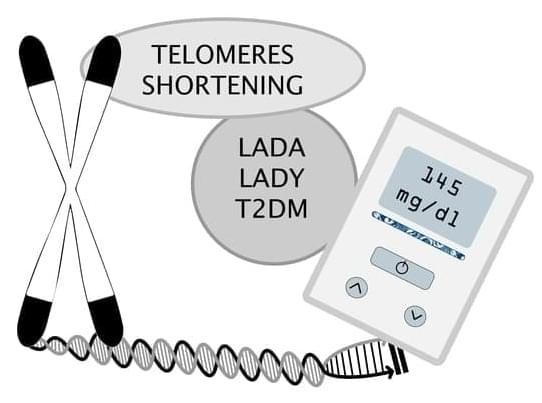
This study aimed to explore the role of telomere length in three different diabetes types: latent autoimmune diabetes of adulthood (LADA), latent autoimmune diabetes in the young (LADY), and type 2 diabetes mellitus (T2DM). A total of 115 patients were included, 72 (62.61%) had LADA, 30 (26.09%) had T2DM, and 13 (11.30%) had LADY. Telomere length was measured using real-time Polymerase Chain Reaction. For statistical analysis, we used the ANOVA test, X2 test, and the Mann–Whitney U test. Patients with T2DM had higher BMI compared to LADA and LADY groups, with a BMI average of 31.32 kg/m2 (p = 0.0235). While the LADA group had more patients with comorbidities, there was not a statistically significant difference (p = 0.3164, p = 0.3315, p = 0.3742 for each of the previously mentioned conditions).

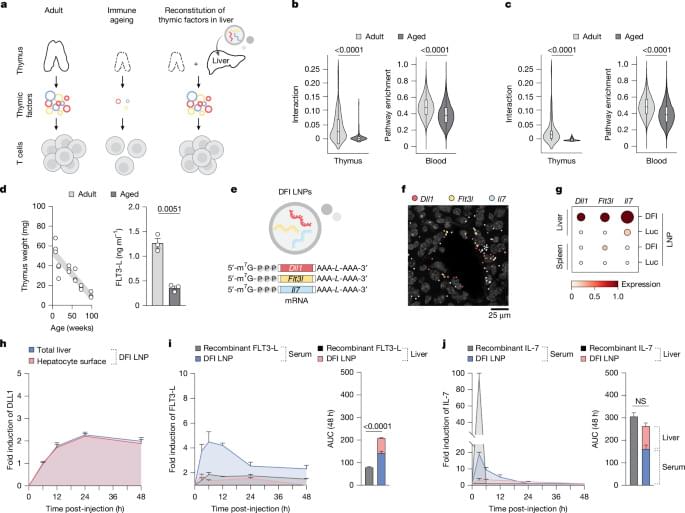
As people age, their immune systems decline. But a new study suggests a way to rejuvenate immune function: Stimulating the liver to produce some signals ordinarily generated by the thymus can reverse age-related declines in T-cell populations.
MIT and Broad Institute researchers found a way to overcome age-related immune system decline by temporarily programming liver cells to take over the maturation of T cells. Using mRNA, the researchers were able to rejuvenate the immune system, in a study of mice.
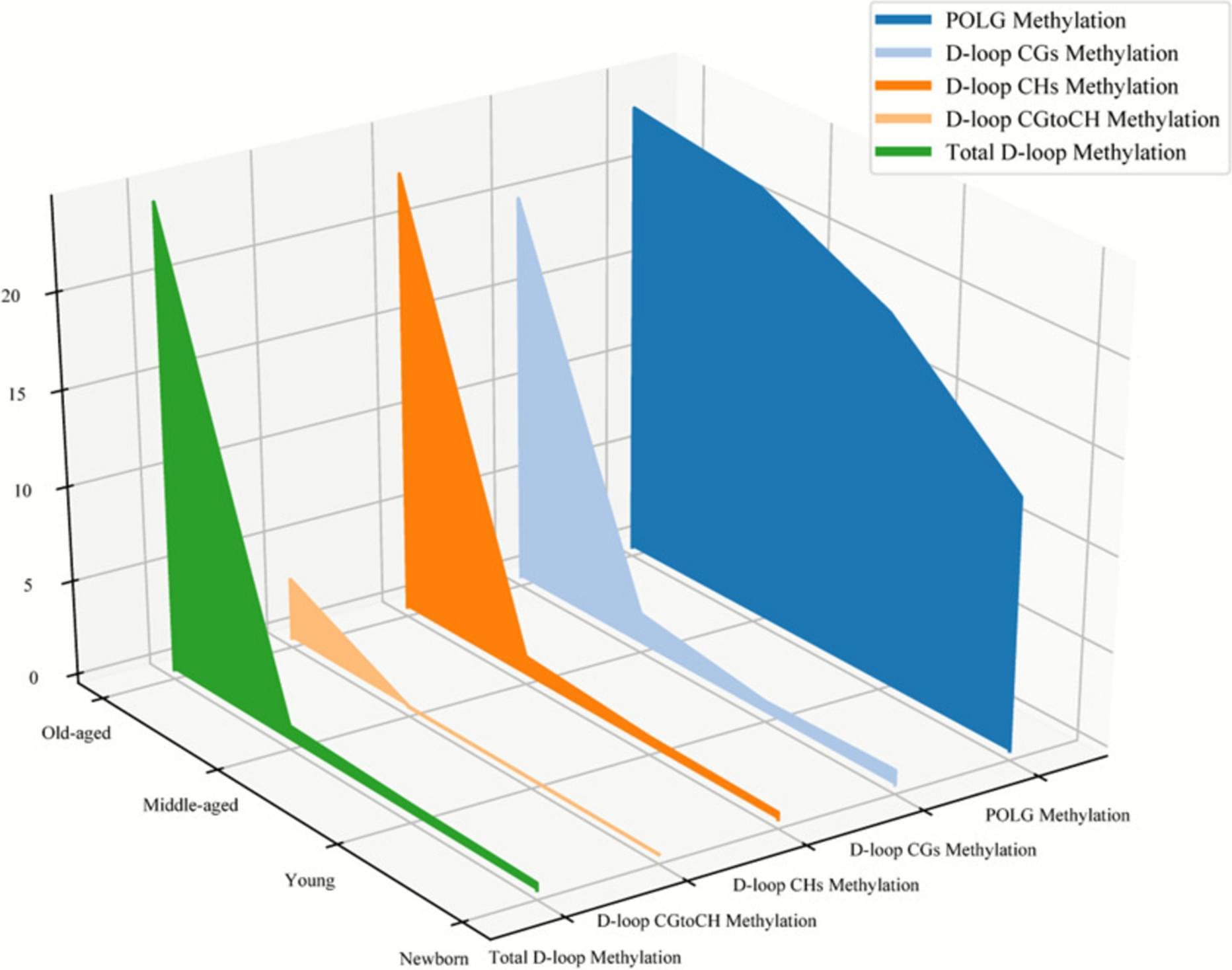
Growing evidence shows that epigenetic modification and mitochondrial dysfunction are hallmarks of aging and are associated with the development of a wide range of age-related diseases. Mitochondrial biogenesis, which is marked by mitochondrial DNA copy number (mtDNAcn), is one of the major regulations of mitochondrial function by a set of transacting elements, including mitochondrial DNA polymerase gamma (POLG), working on the mtDNA control region. In this study, we investigated the mtDNAcn and the methylation status at both mtDNA control and POLGA promoter regions in human blood cells from individuals with a wide range of ages. A total of 119 blood samples were collected, including 24 umbilical cord blood samples from newborns and 95 peripheral blood samples from individuals aged 18 to 96 years.
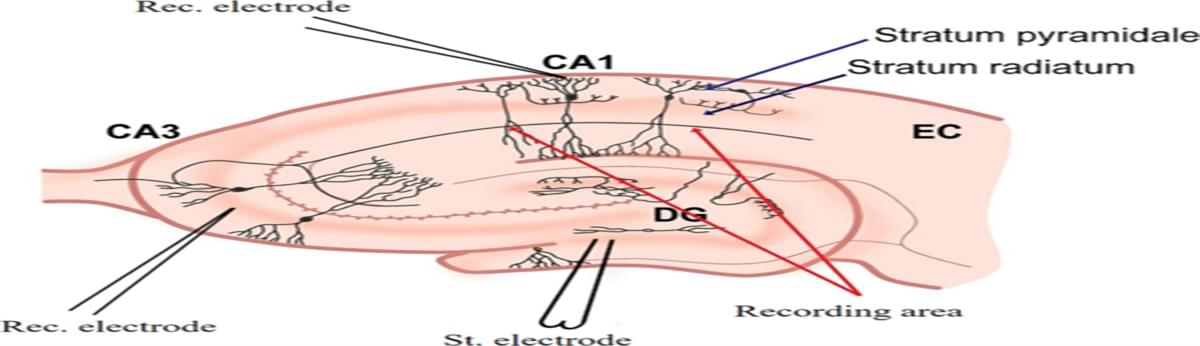
Isting gap in neuromorphic engineering by mimicking biological neuron dynamics and realizing effective clinical applications to promote functional recovery and quality of life enhancement in patients with brain injury. The novel neuromorphic engineering approaches leverage the dynamic behavior of brain neurons, incorporating electronic circuits that emulate neuronal dynamics. A basic configuration involves a neural model designed to mimic the dynamics of a living neuron, with the potential to replace damaged brain tissue when implanted, thus restoring signal propagation. An enhanced configuration integrates a closed-loop system, wherein the feedback signal from biological neurons synchronizes the artificial neuron with its living counterpart, allowing continuous self-adjustment of system parameters and promoting a neuro-autogenerative regime.