Here we look at the recent paper from the PEARL trial where rapamycin is being tested in healthy older adults for its safety and impact on markers of health…
Category: health – Page 74
World’s first “nonstop beating heart” transplant is a medical breakthrough
For the first time, surgeons have successfully performed a remarkable new heart transplant in which the donor organ never skips a beat in the process, reducing the damage that can occur during such a complex operation. It ushers in a new era of more successful heart transplant surgery.
A team of surgeons at the National Taiwan University Hospital (NTUH) in Taipei undertook the revolutionary operation, during which the donor heart continues beating between the organ removal and transplantation stages. Traditionally, the donor heart would be removed and preserved in cold storage to reduce its workload – during this stage, it’s considered “ischemic time,” or the period during which the organ is cut off from blood supply. This comes with the risk of heart damage and rejection once it’s transplanted into a recipient.
When the heart is deprived of blood, ischemia – a shortage of oxygen – can damage its muscle tissue, or myocardium, reducing function and health once it is transplanted. While an organ set for transplant rarely endures more than a few hours in ischemic time, it can still lead to myocardial damage.
Visualizing the measles virus reveals a potential treatment
Measles, a highly contagious viral infection, continues to pose a significant public health threat worldwide. Despite the availability of effective vaccines, outbreaks persist, particularly in regions with low immunization rates. In 2023, the World Health Organization observed up to a 30-fold increase in measles cases in Europe. There are currently no treatment options for measles. Instead, patients must allow the virus to take its course and let the immune system naturally clear the infection.
Erica Ollmann Saphire, a structural biologist, and her research team at the La Jolla Institute for Immunology uncovered the structure of the measles glycoprotein and engineered a neutralizing antibody against it. This therapy could be implemented to manage measles outbreaks worldwide.
Researchers uncovered the structure of the measles fusion glycoprotein and identified a neutralizing antibody capable of decreasing its virulence.
Artificial Intelligence is Reshaping Healthcare
As of October 2024, 3,000 patients had used Piction’s clinic. So far, it is available in Connecticut, Florida, Massachusetts, New Hampshire and Washington. The service is covered by several major insurance companies, or patients can pay $119 out-of-pocket for each consultation.
Eleni Linos, a professor of dermatology and epidemiology who directs the Stanford Center for Digital Health, and who has no connection with Piction, says: “I’m really optimistic about how this technology can help patients get the best care they can get, while at the same time helping doctors.” — Esther Landhuis.

New Pill Form of Semaglutide Shows Major Benefits for People With Diabetes
Both the injectable and oral forms of semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, have recently gained attention for their effectiveness in managing weight gain, high blood sugar, and even reducing alcohol cravings.
A new clinical trial, co-led by endocrinologist and diabetes specialist John Buse, MD, PhD, and interventional cardiologist Matthew Cavender, MD, MPH, at the UNC School of Medicine, has demonstrated that the oral form of semaglutide significantly lowers the risk of cardiovascular events in individuals with type 2 diabetes, atherosclerotic cardiovascular disease.
Cardiovascular disease (CVD) encompasses a range of disorders affecting the heart and blood vessels, including coronary artery disease, heart attack, stroke, and hypertension. These conditions are primarily driven by atherosclerosis, a process where plaque builds up in the arterial walls, leading to narrowed or blocked arteries. Risk factors include smoking, unhealthy diet, lack of exercise, obesity, and genetic predisposition. CVD remains a leading cause of global mortality, emphasizing the importance of lifestyle changes, medical interventions, and preventive measures in managing and reducing the risk of heart-related illnesses.
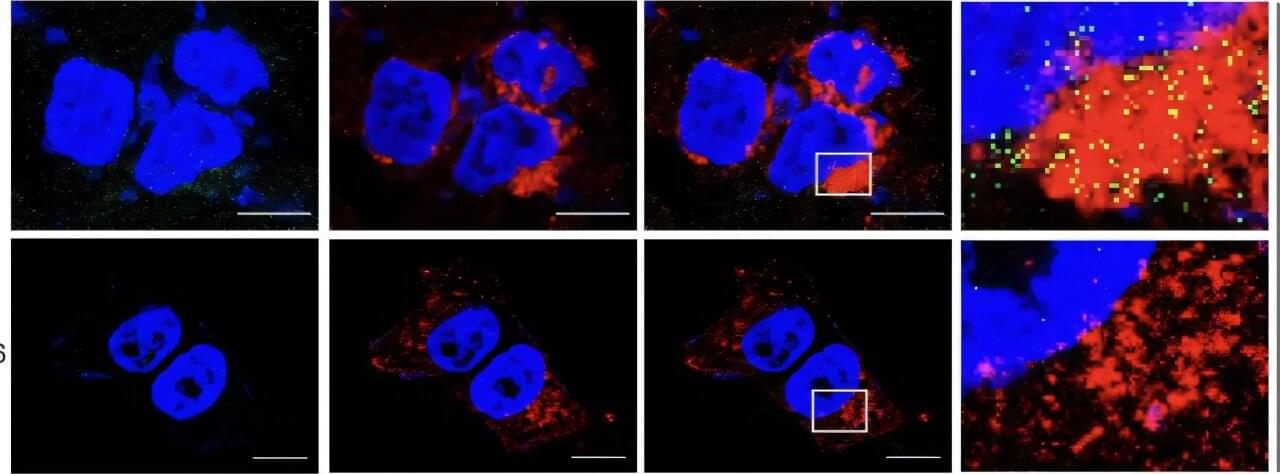
Scientists hack cell entry to supercharge cancer drugs
A new discovery could pave the way for more effective cancer treatment by helping certain drugs work better inside the body. Scientists at Duke University School of Medicine, University of Texas Health Science Center at San Antonio, and University of Arkansas have found a way to improve the uptake of a promising class of cancer-fighting drugs called PROTACs, which have struggled to enter cells due to their large size.
The new method works by taking advantage of a protein called CD36 that helps pull substances into cells. By designing drugs to use this CD36 pathway, researchers delivered 7.7 to 22.3 times more of the drug inside cancer cells, making the treatment up to 23 times more potent than before, according to the study published April 17 in Cell.
Data from mouse studies shows this enhanced uptake led to stronger tumor suppression without making the drugs harder to dissolve or less stable.
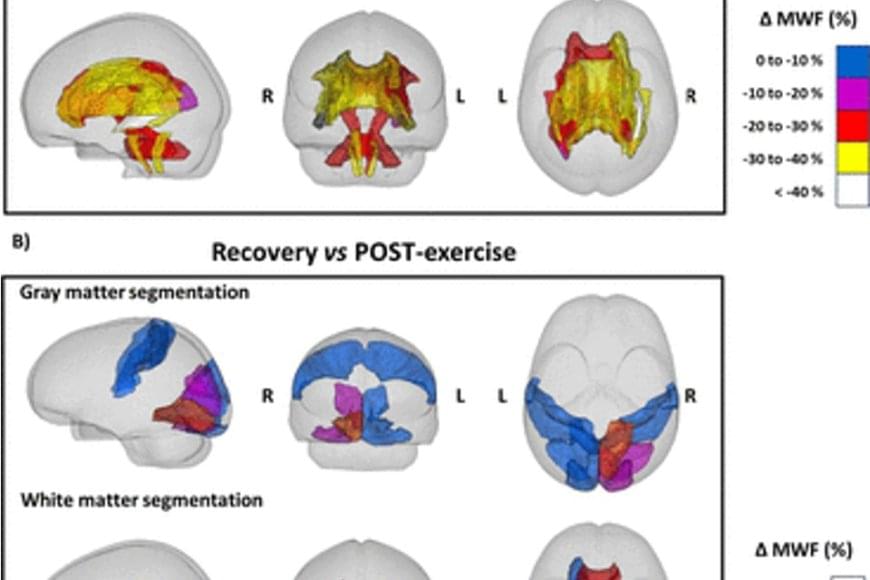
The brain utilizes myelin during endurance exercise
Exercise for a long period of time forces the human body to resort to its energy reserves. When running a marathon, for example, the body mainly consumes carbohydrates, such as glycogen, as a source of energy, but it resorts to fats when the glycogen in the muscles is used up. Myelin, which surrounds neurons in the brain and acts as an electrical insulator, mainly comprises lipids, and previous research in rodents suggests that these lipids can act as an energy reserve in extreme metabolic conditions.
A study conducted by researchers shows that people who run a marathon experience a decrease in the amount of myelin in certain regions of the brain. According to the study published by Nature Metabolism, this effect is completely reversed two months after the marathon.
The researchers used magnetic resonance imaging to obtain images of the brains of ten marathon runners (eight men and two women) before and 48 hours after the 42-kilometre race. Likewise, the researchers took images of the brains of two of the runners two weeks after the race, and of six runners two months after the race as a follow-up.
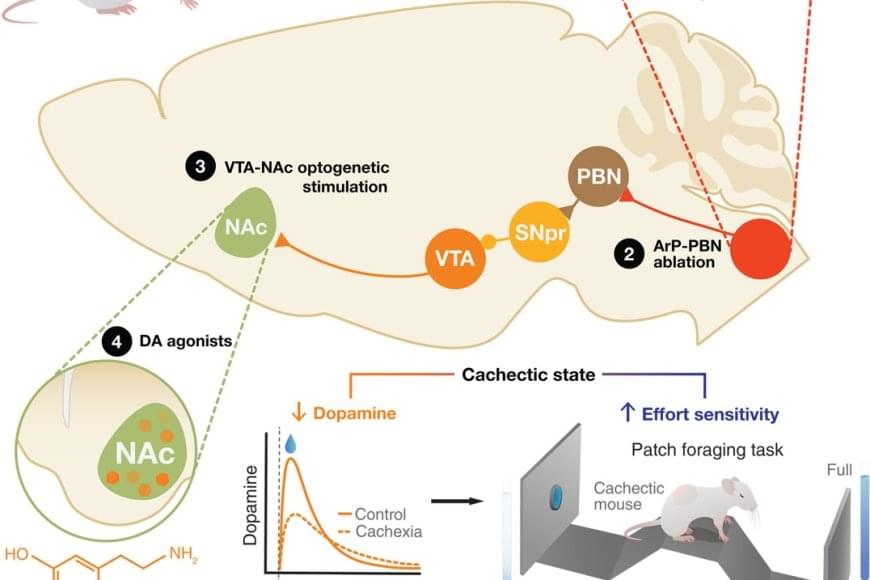
A neuroimmune circuit mediates cancer cachexia-associated apathy
The fatigue and lack of motivation that many cancer patients experience near the end of life have been seen as the unavoidable consequences of their declining physical health and extreme weight loss. But new research challenges that long-held assumption, showing instead that these behavioral changes stem from specific inflammation-sensing neurons in the brain.
In a study published in Science, the researchers report that they identified a direct connection between cancer-related inflammation and the loss of motivation characteristic of advanced cancer. Studying mice with cancer-linked cachexia, a condition typical of the disease that leads to muscle wasting and weight loss, they discovered a previously unrecognized pathway in the brain. This pathway senses inflammation and actively suppresses dopamine — a key driver of motivation — resulting in apathy and loss of drive.
Blocking the pathway restored motivation, even though the cancer and weight loss continued. This indicates that apathy can be treated separately from the disease itself.
The Breakthrough Blood Test for Alzheimer’s Disease
The p-Tau217 biomarker is one of the most exciting advances in neurology for decades, giving us a new opportunity to accurately predict and potentially prevent (or at least substantially delay) MCI and Alzheimer’s. That it rises so early in the course of the disease—which incubates over 20 years—gives us a long runway of opportunity to intervene, be it with lifestyle factors or drugs. I now refer to the former as lifestyle plus because it is no longer just about the details of diet, exercise and sleep. There are several other dimensions of modifiable factors.
An APOE4 allele or a polygenic risk score for Alzheimer’s tests are binary. They only tell us if a person has increased risk (yes or no) but not when. It makes a huge difference if that at age 98 or 68. With serial assessment of p-Tau217 (several months or years apart) as part of a comprehensive assessment using multimodal A.I., it is very likely that the temporal plot (see Figures under Question 2 above) can be defined at the individual level. I lay out the blueprint for this and lifestyle plus fully in Super Agers. Individuals with elevated p-Tau217 at high-risk many years before the onset of any symptoms creates a new path for surveillance and prevention. Multiple new drugs are in the pipeline to be part of a prevention program.
Even though it intuitively appears to be the case, more work needs to be done to determine whether lowering one’s p-Tau217 will alter the brain plaque progression and be seen as a disease-modifier. Clearly there is now a hunt for even better blood tests that may one day supersede p-Tau217 or be in a panel with it.