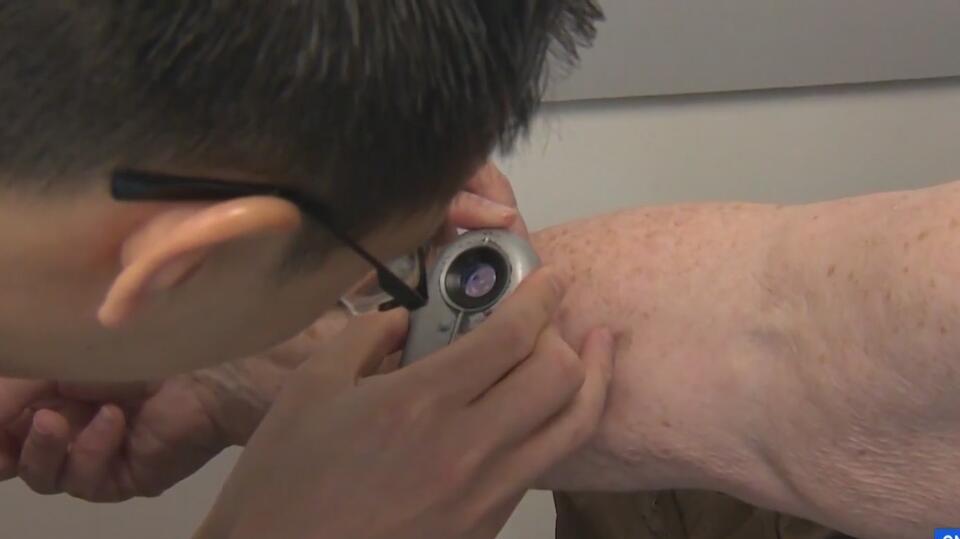
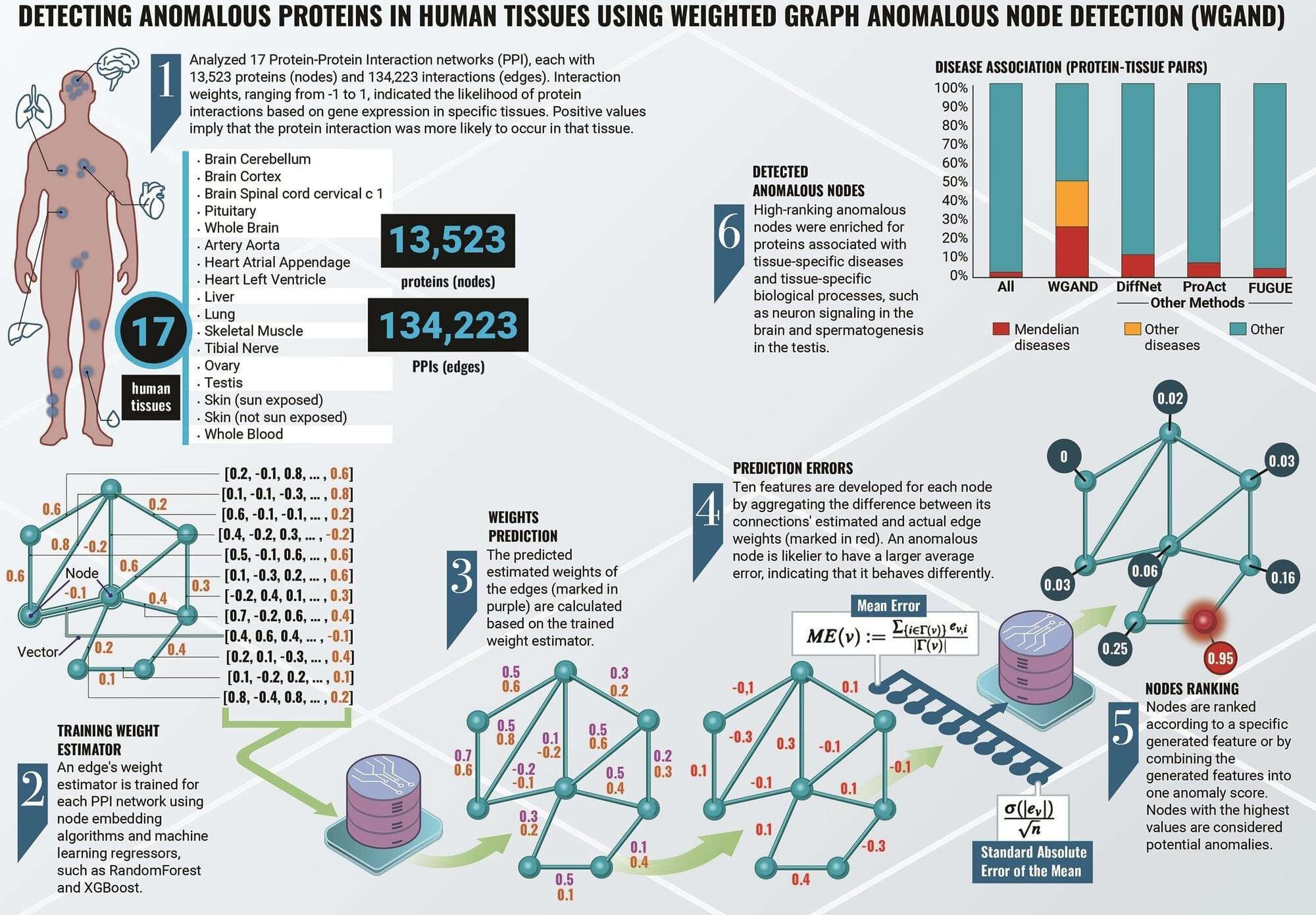
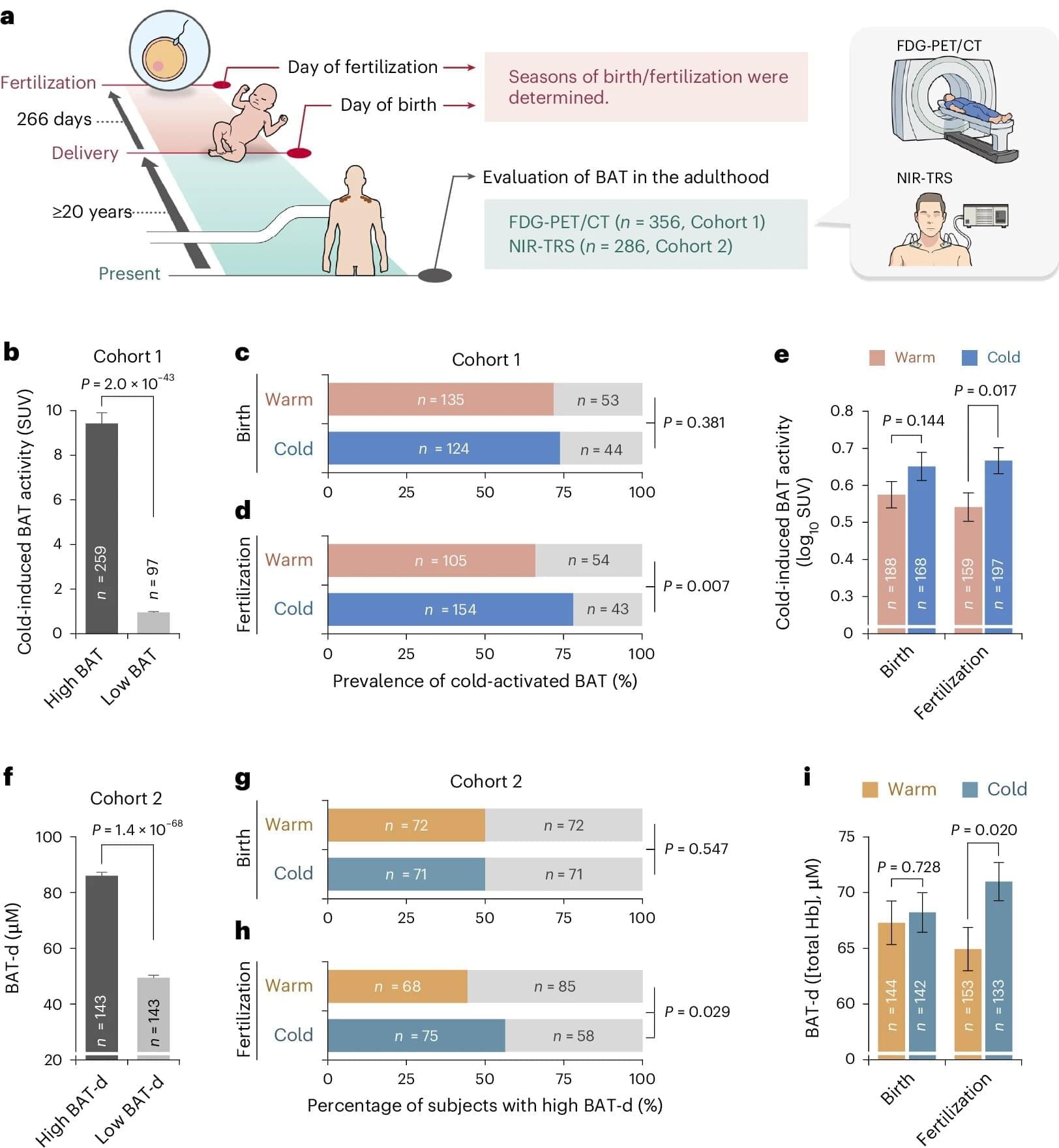
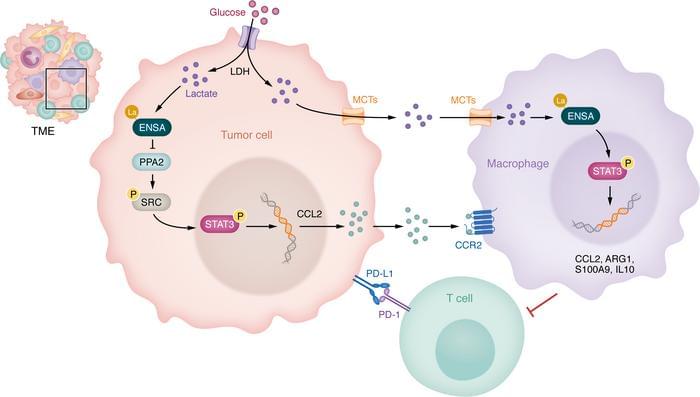
Health care providers can use small devices to hover over moles or lesions and immediately check for common skin cancers, such as melanoma and basal cell carcinoma.
The most significant benefit is that health care professionals who do not specialize in dermatology could perform these checks during a routine visit, making early detection easier and quicker.
Skin cancer is the most common form of cancer in the United States, with one in five Americans expected to be affected in their lifetime, according to the City of Hope Cancer Center.