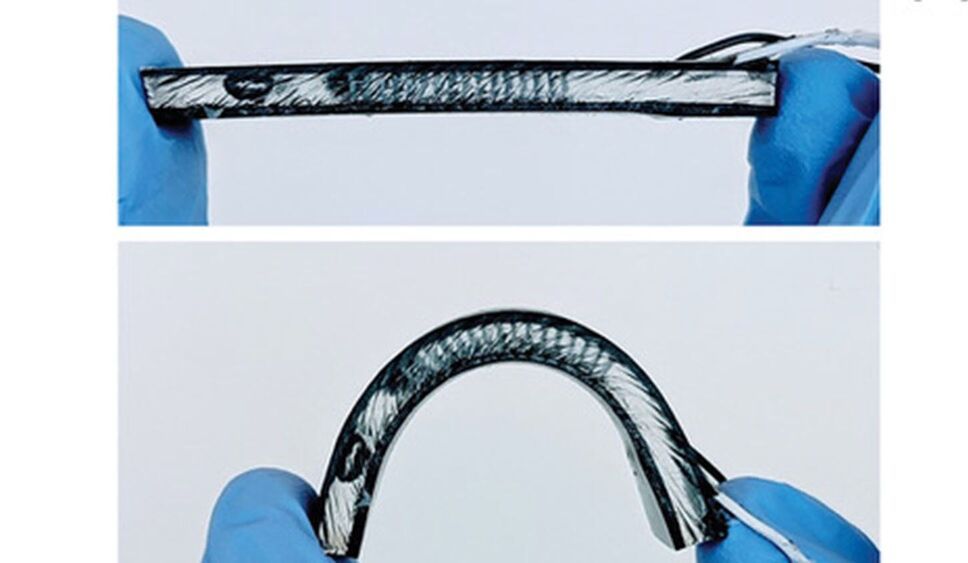
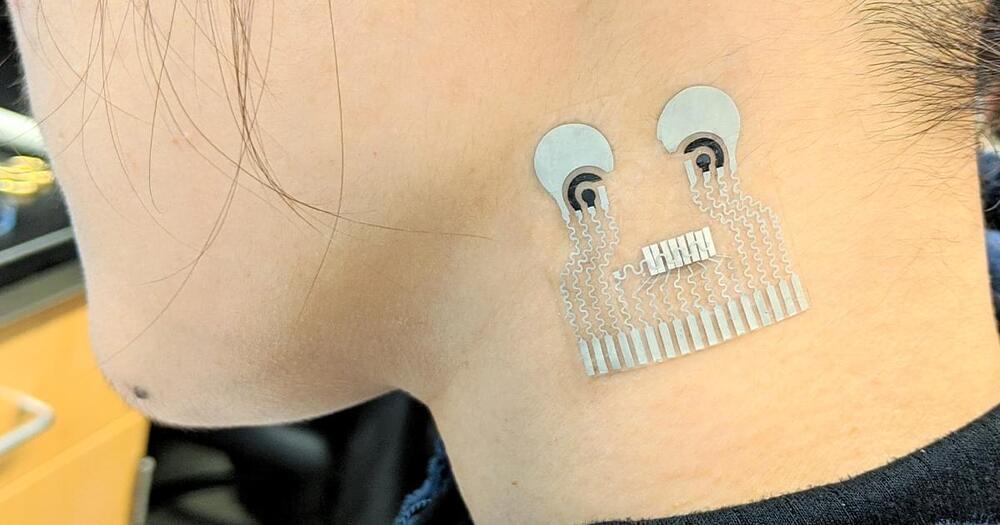
North Carolina State University engineers continue to improve the efficiency of a flexible device worn on the wrist that harvests heat energy from the human body to monitor health.
In a paper published in npj Flexible Electronics, the NC State researchers report significant enhancements in preventing heat leakage in the flexible body heat harvester they first reported in 2017 and updated in 2020. The harvesters use heat energy from the human body to power wearable technologies —think of smart watches that measure your heart rate, blood oxygen, glucose and other health parameters—that never need to have their batteries recharged. The technology relies on the same principles governing rigid thermoelectric harvesters that convert heat to electrical energy.
Flexible harvesters that conform to the human body are highly desired for use with wearable technologies. Mehmet Ozturk, an NC State professor of electrical and computer engineering and the corresponding author of the paper, mentioned superior skin contact with flexible devices, as well as the ergonomic and comfort considerations to the device wearer, as the core reasons behind building flexible thermoelectric generators, or TEGs.