Newly discovered genes could make the powerful anticancer drug, Taxol, cheaper and more sustainable to produce.


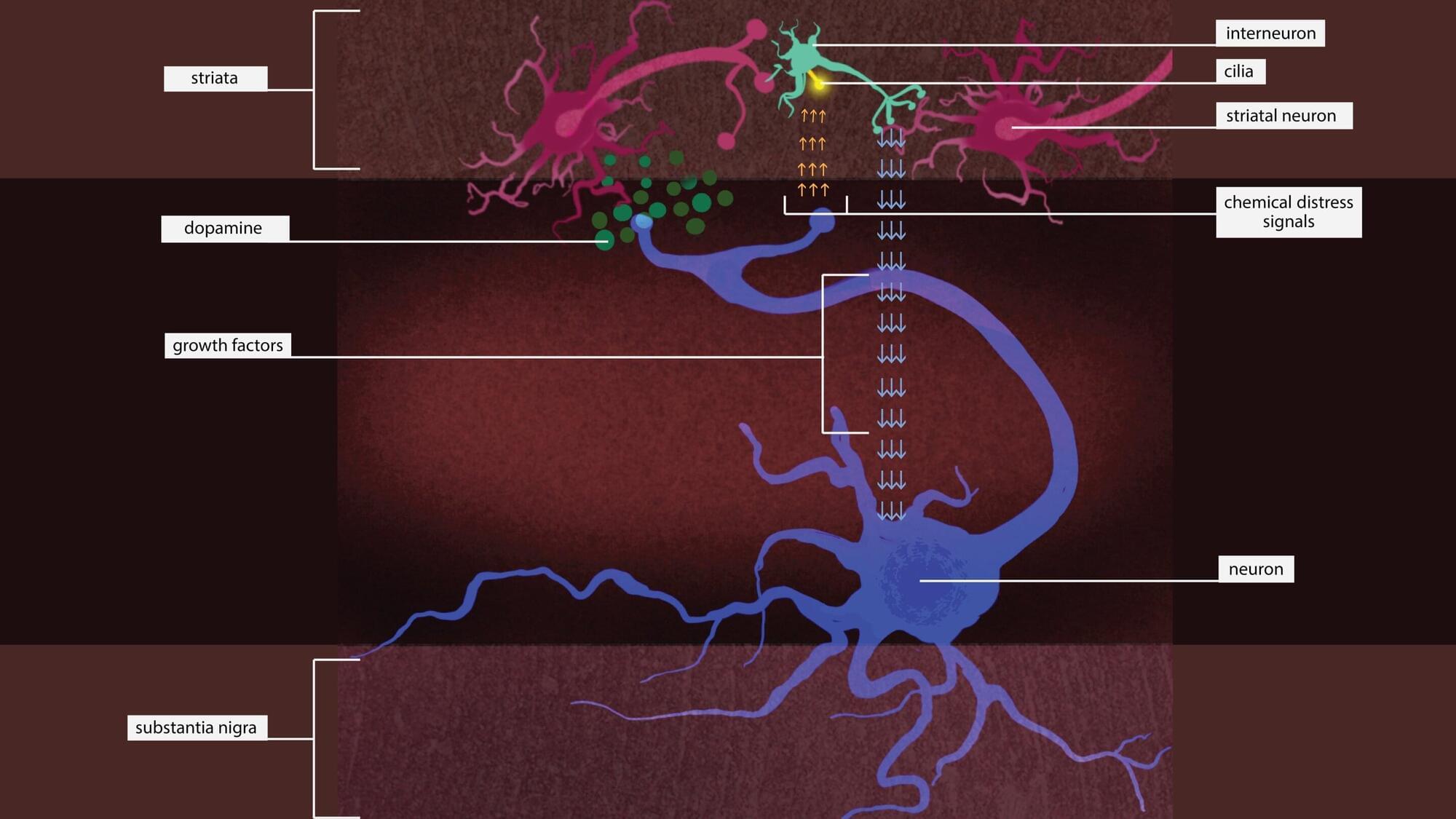
Putting the brakes on an enzyme might rescue neurons that are dying due to a type of Parkinson’s disease that’s caused by a single genetic mutation, according to a new Stanford Medicine-led study conducted in mice.
The study has been published in Science Signaling.
The genetic mutation causes an enzyme called leucine-rich repeat kinase 2, or LRRK2, to be overactive. Too much LRRK2 enzyme activity changes the structure of brain cells in a way that disrupts crucial communication between neurons that make the neurotransmitter dopamine and cells in the striatum, a region deep in the brain that is part of the dopamine system and is involved in movement, motivation and decision-making.
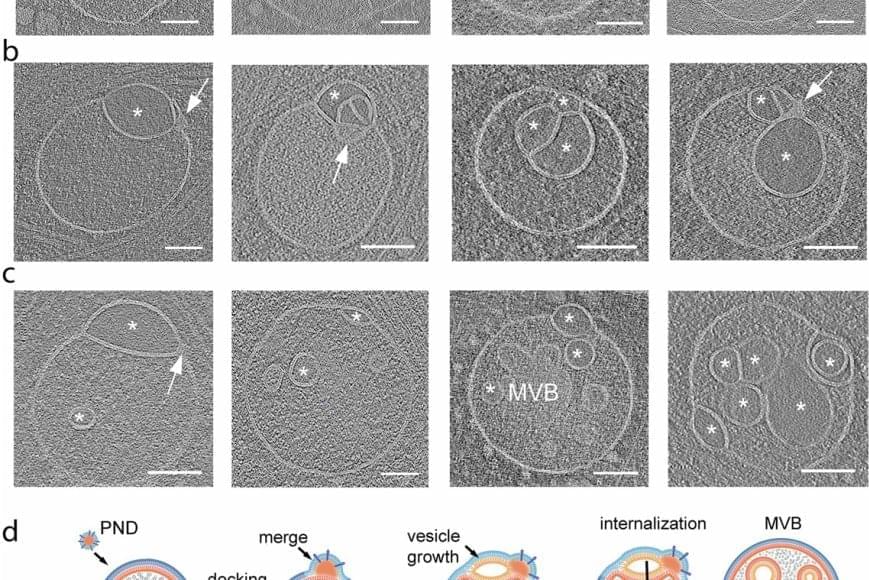
The discovery of an unknown organelle inside our cells could open the door to new treatments for devastating inherited diseases.
The organelle, a type of specialized structure, has been dubbed a “hemifusome” by its discoverers. This little organelle has a big job helping our cells sort, recycle and discard important cargo within themselves, the scientists say. The new discovery could help scientists better understand what goes wrong in genetic conditions that disrupt these essential housekeeping functions.
One such condition is Hermansky-Pudlak syndrome, a rare genetic disorder that can cause albinism, vision problems, lung disease and issues with blood clotting. Problems with how cells handle cargo are at the root of many such disorders.
The scientists believe hemifusomes facilitate the formation of vesicles, tiny blister-like sacs that act as mixing bowls, and of organelles made up of multiple vesicles. This process is critical to cellular sorting, recycling and debris disposal, the researchers report.

A team of researchers at NYU Abu Dhabi has uncovered a key mechanism that helps shape how our brains are wired, and what can happen when that process is disrupted.
In a new study published in Cell Reports, the RNA-MIND Lab at NYU Abu Dhabi, led by Professor of Biology Dan Ohtan Wang, with Research Associate Belal Shohayeb, reveals how a small molecular mark on messenger RNA, called m6A methylation, regulates the production of essential proteins inside growing neurons. This process plays a critical role in the development of axons, the long extensions that neurons use to connect and communicate with each other.
The study shows that this molecular mark controls the production of a protein called adenomatous polyposis coli (APC), which helps organize the internal structure of nerve cells and is needed to locally produce β-actin, a key building block of the cytoskeleton to support axon growth. Importantly, the team also found that genetic mutations linked to autism and schizophrenia can interfere with this process, potentially affecting how the brain develops.

“We need diagnostic methods that are more rapid, reliable and capable of detecting multiple pathogens simultaneously,” explains Thai, who is one of the grant recipients of the 2023–24 Seegene Open Innovation Programme. “We also need to ensure that these tools are widely accessible and effectively integrated into clinical and laboratory workflows.”
Polymerase chain reaction (PCR) tests, which rose to worldwide fame during the COVID-19 pandemic, amplify tiny snippets of genetic material from pathogens in samples to levels that can be easily detected.
Seegene, a molecular diagnostics company based in Seoul, South Korea, has developed ‘syndromic multiplex PCR’ technology capable of detecting up to 14 pathogens in a single test.
Year 2013 face_with_colon_three Basically this is the light based nanotransfection version that can eventually be put on a simple smartphone or smartwatch that can be an entire hospital in one touch healing the entire body in one touch or just areas that need healing.
Antkowiak, M., Torres-Mapa, M., Witts, E. et al. Sci Rep 3, 3,281 (2013). https://doi.org/10.1038/srep03281

Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/

A new gene therapy delivery device could let hospital pharmacies make personalized nanomedicines to order. This democratized approach to precision medicine, as published in Frontiers in Science, could revolutionize how hospitals treat rare diseases, even in low-resource settings.
Rare diseases affect millions worldwide, yet the one-size-fits-all model of drug development leaves patients with few treatment options. Now a European research project called NANOSPRESSO aims to tip the balance in patients’ favor by boosting access to low-cost bespoke gene and RNA therapies.
The prototype NANOSPRESSO device combines two proven technologies— nucleic acid therapeutics and lipid nanoparticles—into a portable manufacturing unit. Hospital pharmacists could use the unit to prepare sterile, injectable nanomedicines tailored to the specific genetic abnormality causing the patient’s condition, bypassing the need for centralized drug production.
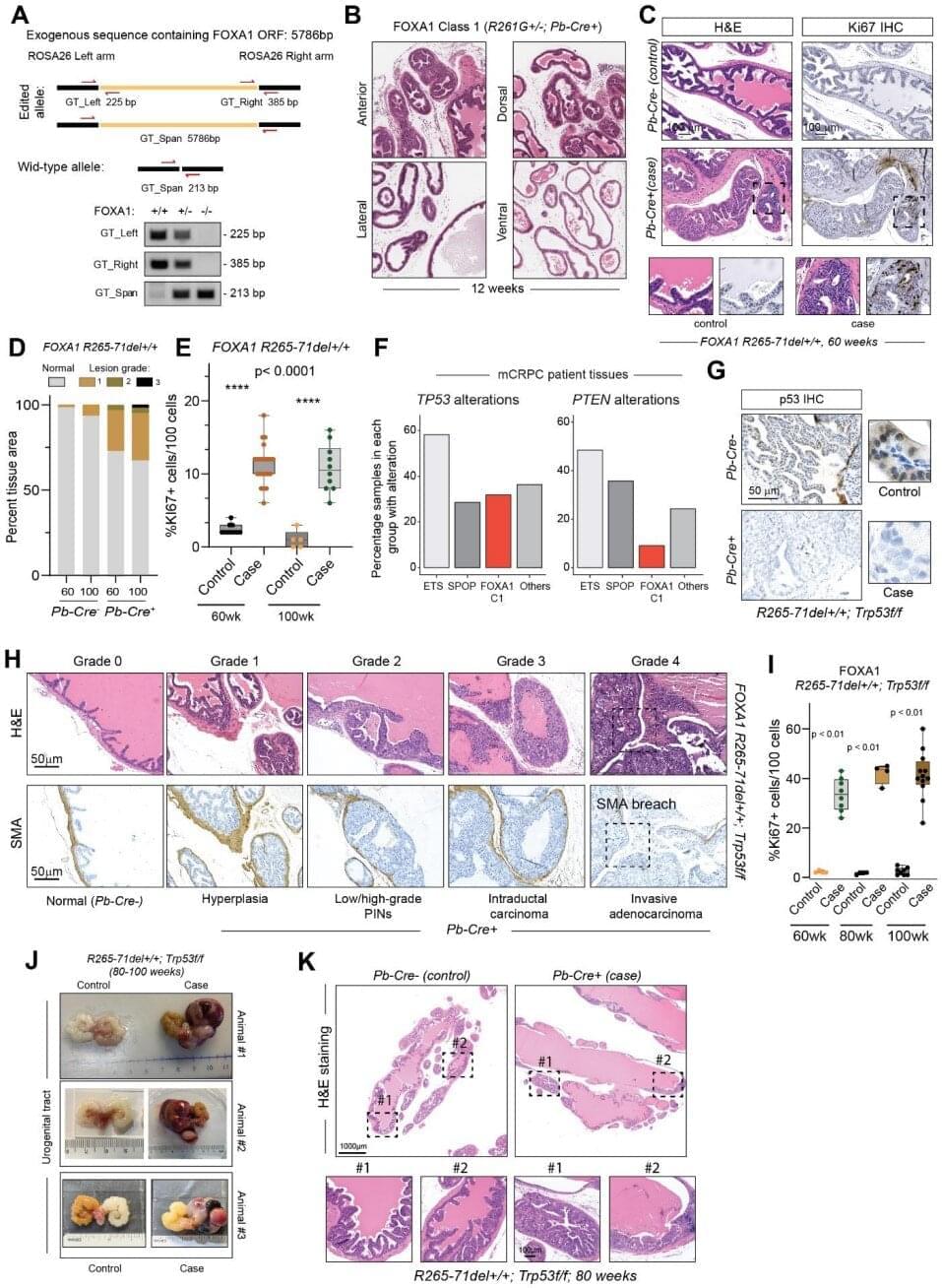
A new study from the University of Michigan Rogel Health Cancer Center, published in Science, sheds light on how two distinct classes of mutations in the FOXA1 gene—commonly altered in prostate cancer—drive tumor initiation formation and therapeutic resistance.
FOXA1, a key transcription factor that facilitates androgen receptor binding to DNA, is mutated in 10–40% of hormone-dependent prostate cancers. While common, the exact ways these mutations alter cancer cells have remained elusive—until now.
Rogel researchers, including Arul Chinnaiyan, M.D., Ph.D., S.P. Hicks Endowed Professor of Pathology and Urology, and Abhijit Parolia, Ph.D., Rogel Fellow and Assistant Professor of Pathology, used mouse models to understand the mechanisms underlying two major classes of FOXA1 mutations.