Scientists have been analyzing certain animals living within the CEZ for years, including bacteria, rodents, and even birds. One study back in 2016 found that Eastern tree frogs (Hyla orientalis), which are usually a green color, were more commonly black within the CEZ. The biologists theorize that the frogs experienced a beneficial mutation in melanin—pigments responsible for skin color—that helped dissipate and neutralize some of the surrounding radiation.
This made scientists ponder: could something similar be happening to Chernobyl’s wild dogs?
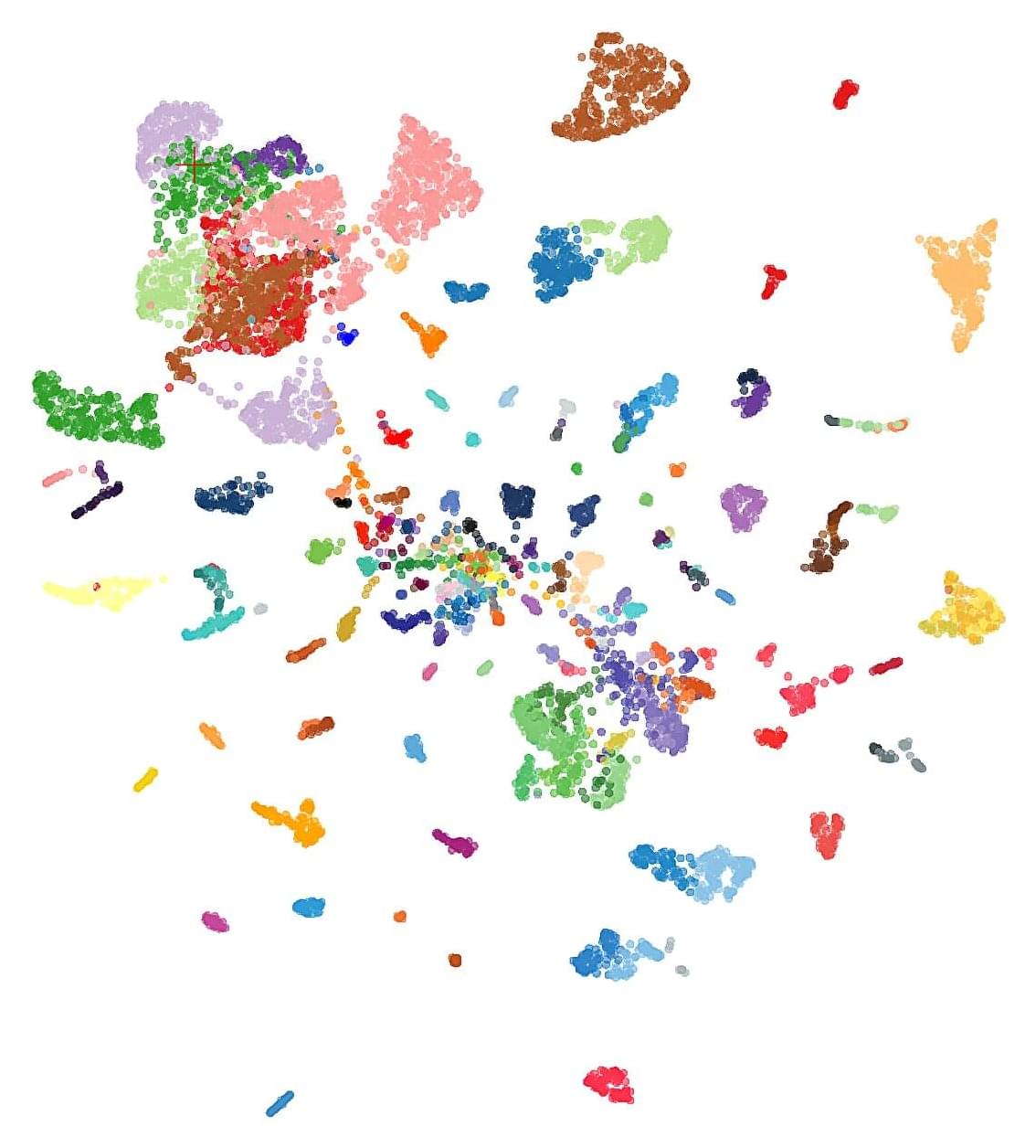
The study uncovered that the feral dogs living near the Chernobyl Power Plant showed distinct genetic differences from dogs living only some 10 miles away in nearby Chernobyl City. While this may seem to heavily imply that these dogs have undergone some type of rapid mutation or evolution due to radiation exposure, this study is only a first step in proving that hypothesis.