This AI system can analyze up to one million DNA letters at once, predicting how tiny changes in noncoding regions trigger everything from cancer to rare genetic disorders—and potentially revolutionizing personalized medicine


IN A NUTSHELL 🌾 The Moon-Rice project is developing “super-dwarf” rice to support long-duration space missions and extreme Earth environments. 🛰️ Led by the Italian Space Agency, the project involves collaboration between three Italian universities specializing in rice genetics, crop physiology, and space crop production. 🔬 The research focuses on using CRISPR-Cas technology to create
Researchers have uncovered the types of bacteria, viruses and parasites that plagued ancient humans across Europe and Asia as far back as 37,000 years ago.
An international team, including researchers from the University of Copenhagen, Denmark, Lund University, Sweden, Curtin University, Australia, has created an archaeogenetic-based map of human pathogens across both time and geography.
“Infectious diseases have had devastating effects on human populations throughout history, but important questions about their origins and past dynamics remain,” the authors write.

Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
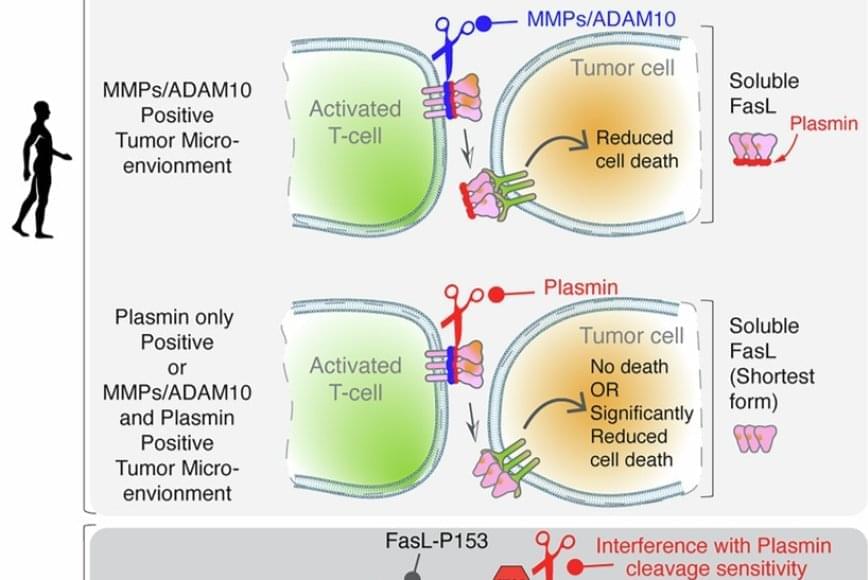
FasL is an immune cell membrane protein that triggers a programmed cell death called apoptosis. Activated immune cells, including CAR-T cells made from a patient’s immune system, use apoptosis to kill cancer cells.
The team discovered that in human genes, a single evolutionary amino acid change — serine instead of proline at position 153 — makes FasL more susceptible to being cut and inactivated by plasmin.
Plasmin is a protease enzyme that is often elevated in aggressive solid tumors like triple negative breast cancer, colon cancer and ovarian cancer.
This means that even when human immune cells are activated and ready to attack the tumor cells, one of their key death weapons — FasL — can be neutralized by the tumor environment, reducing the effectiveness of immunotherapies.
The findings may help explain why CAR-T and T-cell-based therapies can be effective in blood cancers but often fall short in solid tumors. Blood cancers often do not rely on plasmin to metastasize, whereas tumors like ovarian cancer rely heavily on plasmin to spread the cancer.
Significantly, the study also showed that blocking plasmin or shielding FasL from cleavage can restore its cancer-killing power. That finding may open new doors for improving cancer immunotherapy.

UCSF Benioff Children’s Hospital Oakland is enrolling patients in an innovative clinical trial that seeks to cure sickle cell disease. The trial is the first in the U.S. to apply non-viral CRISPR-Cas9 gene-editing technology in humans to directly correct the genetic mutation that causes the disease.

Using an inexpensive electrode coated with DNA, MIT researchers have designed disposable diagnostics that could be adapted to detect a variety of diseases, including cancer or infectious diseases such as influenza and HIV.
These electrochemical sensors make use of a DNA-chopping enzyme found in the CRISPR gene-editing system. When a target such as a cancerous gene is detected by the enzyme, it begins shearing DNA from the electrode nonspecifically, like a lawnmower cutting grass, altering the electrical signal produced.
One of the main limitations of this type of sensing technology is that the DNA that coats the electrode breaks down quickly, so the sensors can’t be stored for very long and their storage conditions must be tightly controlled, limiting where they can be used. In a new study, MIT researchers stabilized the DNA with a polymer coating, allowing the sensors to be stored for up to two months, even at high temperatures. After storage, the sensors were able to detect a prostate cancer gene that is often used to diagnose the disease.


Nerve cells are not just nerve cells. Depending on how finely we distinguish, there are several hundred to several thousand different types of nerve cell in the human brain according to the latest calculations. These cell types vary in their function, in the number and length of their cellular appendages, and in their interconnections. They emit different neurotransmitters into our synapses and, depending on the region of the brain – for example, the cerebral cortex or the midbrain – different cell types are active.
When scientists produced nerve cells from stem cells in Petri dishes for their experiments in the past, it was not possible to take their vast diversity into account. Until now, researchers had only developed procedures for growing a few dozen different types of nerve cell in vitro. They achieved this using genetic engineering or by adding signalling molecules to activate particular cellular signalling pathways. However, they never got close to achieving the diversity of hundreds or thousands of different nerve cell types that actually exists.
“Neurons derived from stem cells are frequently used to study diseases. But up to now, researchers have often ignored which precise types of neuron they are working with,” says Barbara Treutlein, Professor at the Department of Biosystems Science and Engineering at ETH Zurich in Basel. However, this is not the best approach to such work. “If we want to develop cell culture models for diseases and disorders such as Alzheimer’s, Parkinson’s and depression, we need to take the specific type of nerve cell involved into consideration.”
For the first time, researchers at ETH Zurich have successfully produced hundreds of different types of nerve cell from human stem cells in Petri dishes. In the future, it will thus be possible to investigate neurological disorders using cell cultures instead of animal testing.