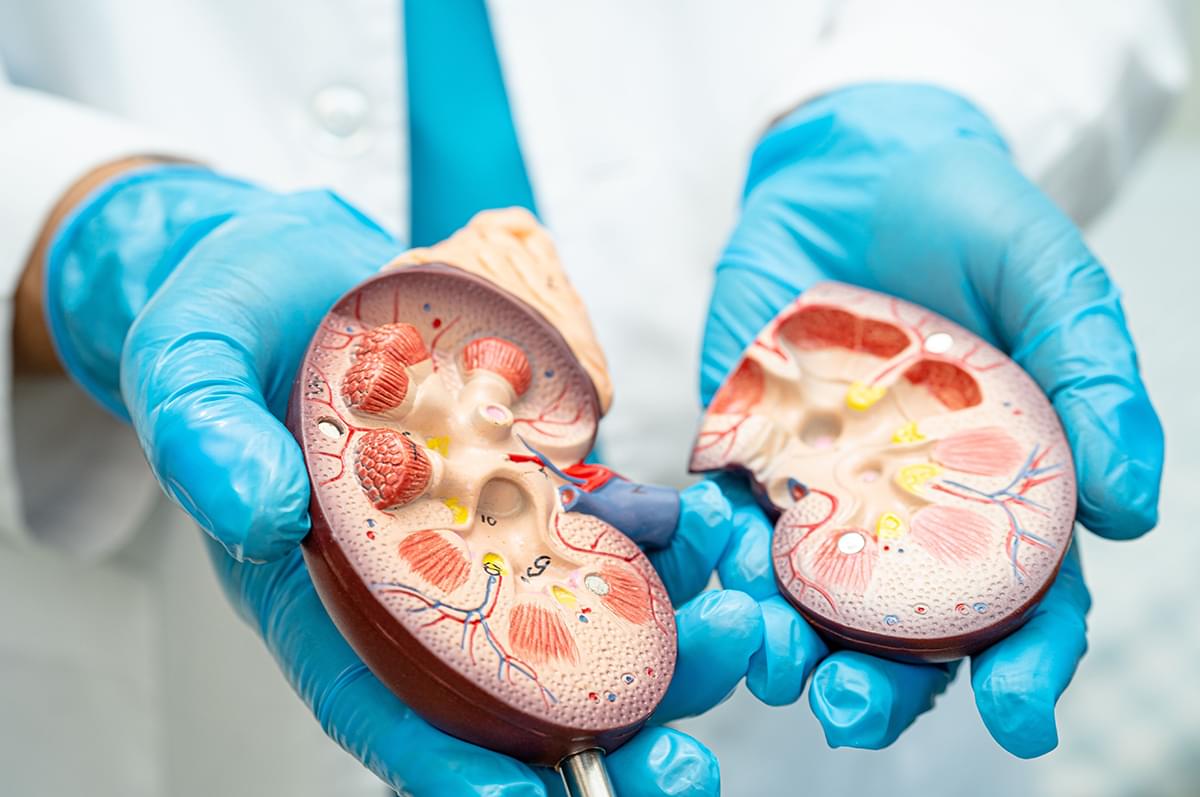
Chronic kidney disease affects an estimated 37 million people in the U.S., and for many, there is no cure. But a new research project at Washington University in St. Louis seeks to change that by uncovering the mechanical basis of kidney cell injury.
To tackle chronic kidney disease, Guy Genin, the Harold and Kathleen Faught Professor of Mechanical Engineering at the WashU McKelvey School of Engineering, and Jeffrey Miner, the Eduardo and Judith Slatopolsky Professor of Medicine in Nephrology at WashU Medicine, teamed up with Hani Suleiman, an assistant professor of medicine at the University of Texas Southwestern Medical Center. The interdisciplinary team, with expertise spanning medicine, cell biology, genetics and engineering, received a five-year $4 million grant from the National Institute of Diabetes and Digestive and Kidney Diseases, part of the National Institutes of Health (NIH).
With the NIH’s support, the team plans to study the mechanobiology of podocytes, specialized cells in the kidney that help filter blood.
Researchers at Washington University in St. Louis have received a $4 million grant to study specialized cells that could help treat kidney disease.