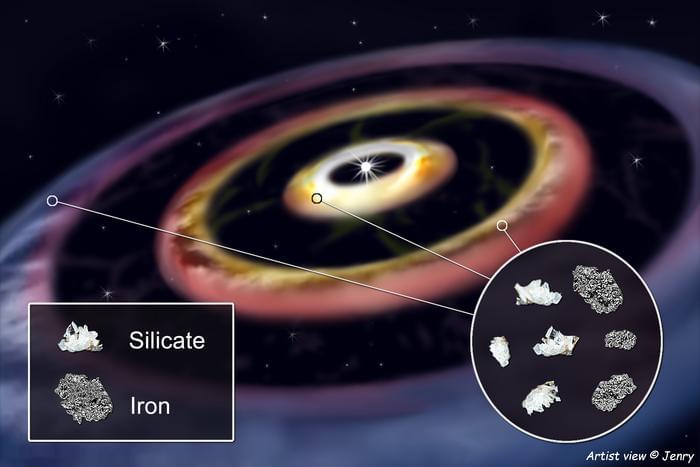
“We think that the HD 144,432 disk may be very similar to the early Solar System that provided lots of iron to the rocky planets we know today,” said Dr. Roy van Boekel.
How did our solar system form and is this process similar in other solar systems throughout the universe? This is what a study published today in Astronomy & Astrophysics hopes to figure out as a team of international researchers used data from the European Southern Observatory’s (ESO) Very Large Telescope Interferometer (VLTI) to analyze the protoplanetary disk around HD 144,432, which is a young star located approximately 500 light-years from Earth. This study holds the potential to not only help researchers better understand the formation and evolution of solar systems, but also gain greater insight into how life could evolve in these systems, as well.
“When studying the dust distribution in the disk’s innermost region, we detected for the first time a complex structure in which dust piles up in three concentric rings in such an environment,” said Dr. Roy van Boekel, who is a scientist at the Max Planck Institute for Astronomy (MPIA) and one of more than three dozen co-authors on the study. “That region corresponds to the zone where the rocky planets formed in the Solar System.”
For context in terms of the distance between the three rings, the innermost ring orbits at the same distance as Mercury, the second farthest ring orbits at the same distance as Mars, and the farthest ring orbits at the same distance as Jupiter.