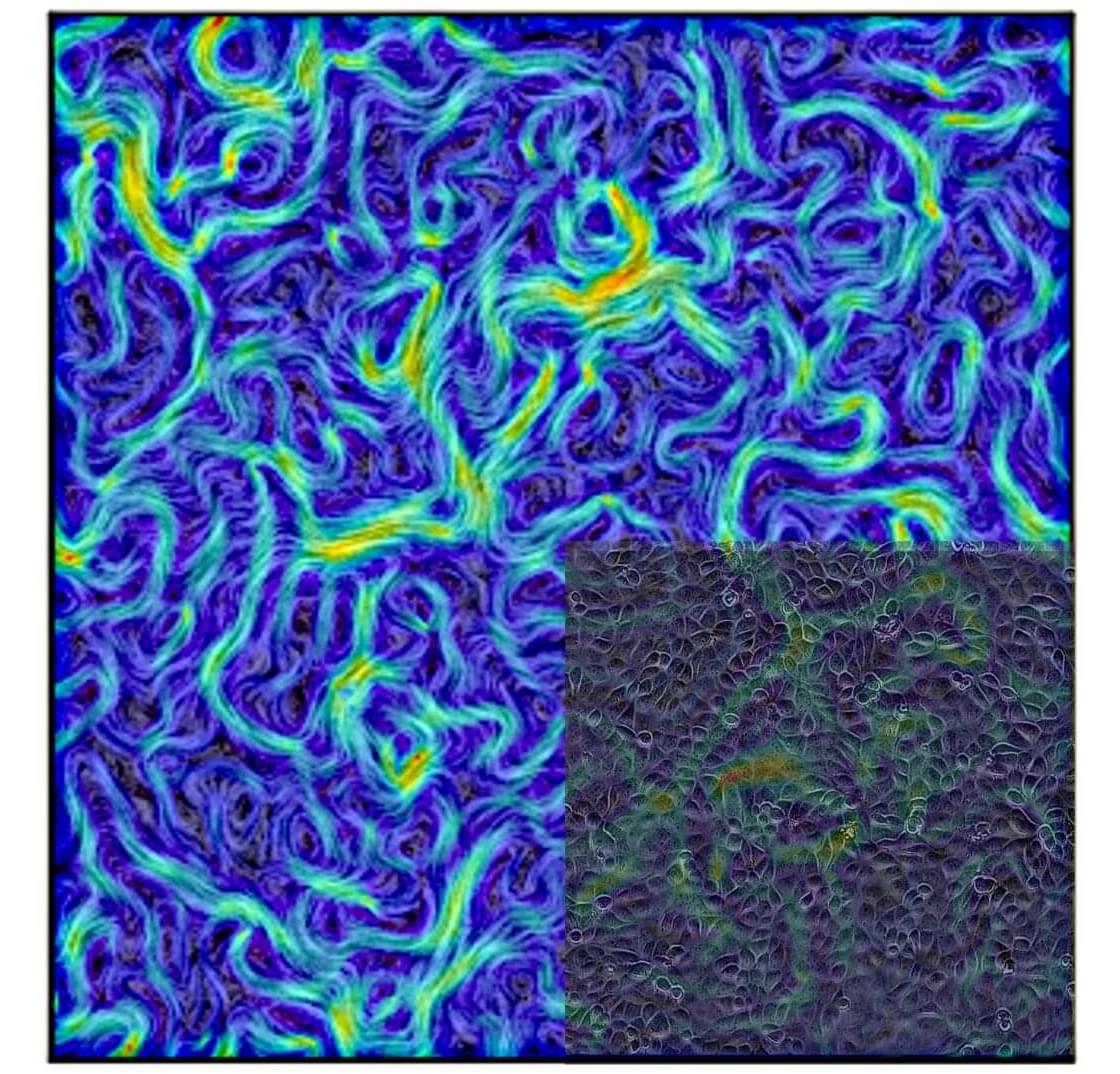
In a new Nature Physics study, researchers have provided evidence of universal conformal invariance in living biological cells. They show that a universal feature in the collective behavior emerges in groups of living cells.
The researchers studied four cellular systems to find evidence of universal conformal invariance. Despite being separated by billions of years of evolution, the researchers found that all four systems generated vortex-like flow patterns with identical statistical properties.
Phys.org spoke to one of the study’s co-authors, Dr. Amin Doostmohammadi, an Associate Professor at the University of Copenhagen.