In relationships, sharing closer spaces naturally deepens the connection as bonds form and strengthen through increasing shared memories. This principle applies not only to human interactions but also to engineering. Recently, an intriguing study was published demonstrating the use of quantum dots to create metasurfaces, enabling two objects to exist in the same space.
Professor Junsuk Rho from the Department of Mechanical Engineering, the Department of Chemical Engineering, and the Department of Electrical Engineering, PhD candidates Minsu Jeong, Byoungsu Ko, and Jaekyung Kim from the Department of Mechanical Engineering, and Chunghwan Jung, a PhD candidate, from the Department of Chemical Engineering at Pohang University of Science and Technology (POSTECH) employed Nanoimprint Lithography (NIL) to fabricate metasurfaces embedded with quantum dots, enhancing their luminescence efficiency. Their research was recently published in Nano Letters (“Printable Light-Emitting Metasurfaces with Enhanced Directional Photoluminescence”).
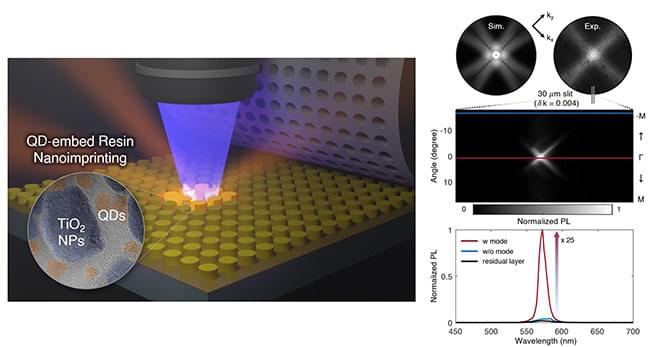
(Left) Schematic diagram of the fabrication of a luminescence-controlled metasurface using the nanoimprint lithography process. (Right) Experiment evaluating the performance of the metasurface’s luminescence control. (Image: POSTECH)