ENeuro: Ringelberg et al. identify a key role for excitatory neuron loss of UBE3A in motor, innate, and sleep behavioral phenotypes of Angelman syndrome model mice.
▶️
AS is a neurodevelopmental disorder with no disease-modifying treatment. However, clinical trials are currently underway using antisense oligonucleotides to unsilence the dormant paternal UBE3A allele, thereby normalizing UBE3A levels (Ionis: NCT05127226; Ultragenyx: NCT04259281). While this approach holds exciting promise and shows efficacy in mouse models (Meng et al., 2015; Milazzo et al., 2021), there is currently scant information regarding the key cell types or brain regions that require UBE3A reinstatement to mitigate core symptoms of AS. This holds particular importance, as effective biodistribution is a key concern in genetic therapies for CNS disorders (Roberts et al., 2020; Jafar-Nejad et al., 2021; Ling et al., 2023), and suboptimal targeting of necessary cell classes could hamper success. Moreover, mouse models of AS require early postnatal Ube3a reinstatement to achieve optimal phenotypic recovery (Silva-Santos et al., 2015; Sonzogni et al., 2020); early intervention could be difficult to achieve in the patient population without a corresponding early diagnosis, meaning many AS individuals are likely beyond the critical window to maximally benefit from UBE3A reinstatement-based therapies. Therefore, additional work is needed to better understand how loss of UBE3A leads to symptoms, as these insights will aid both in understanding the cell types that must be targeted for optimal genetic interventions and in developing alternative therapeutic options.
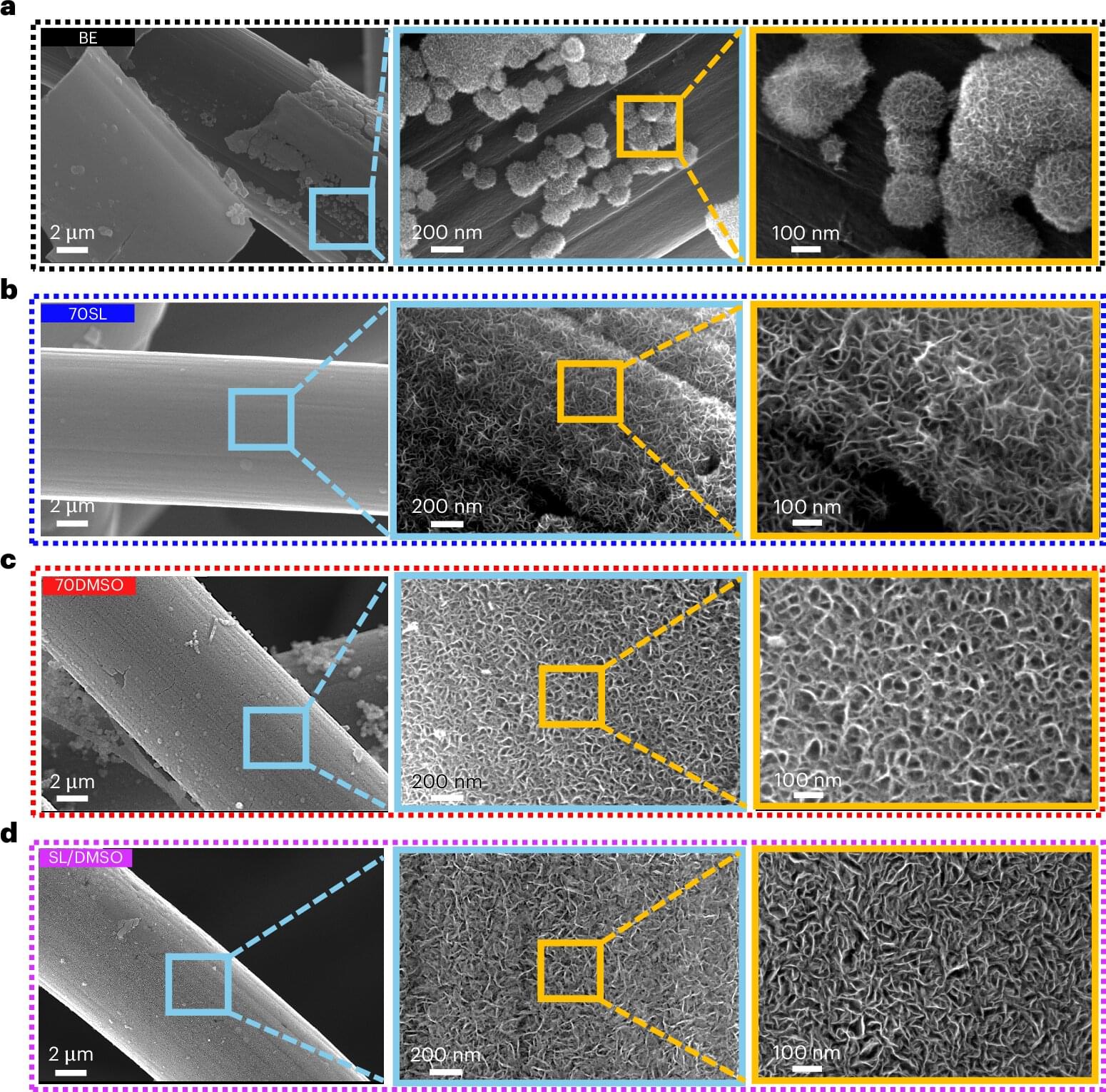
Our laboratory’s previous work identified an outsized role of GABAergic loss of UBE3A in hyperexcitability phenotypes. GABAergic loss of UBE3A drives increased delta power on cortical EEG (Judson et al., 2016), a phenotype that correlates with the severity of a range of symptoms in AS individuals (Hipp et al., 2021; Ostrowski et al., 2021). Further, mice with Ube3a deleted from GABAergic neurons show decreased threshold to chemically and acoustically driven seizures, and they also exhibit spontaneous behavioral seizures, a phenotype not observed in AS model mice on a C57BL/6J background (Judson et al., 2016; Gu et al., 2019). These data forewarn that UBE3A reinstatement in a manner biased to glutamatergic neurons could potentially worsen epilepsy-related symptoms and highlight the importance of studying the neuronal populations regulating other behaviors.
Based on the exaggerated role of GABAergic neurons in AS seizure phenotypes, we predicted that GABAergic deletion of Ube3a would underlie a broad range of behavioral phenotypes in AS mice. In the present study, we instead found a larger role of Ube3a deletion from glutamatergic neurons in motor coordination, measured by rotarod and open field behavior, and innate species-specific behaviors such as marble burying. Furthermore, glutamatergic loss of UBE3A appears to mediate alterations in sleep patterning and induces some sleep fragmentation, while UBE3A loss from GABAergic neurons only caused fragmented sleep. Interestingly, glutamatergic reinstatement of Ube3a also rescued the decreased REM sleep observed in AS mice, as estimated by the PiezoSleep system. While this study identified some roles of GABAergic neurons in nest building behavior and sleep fragmentation, our data largely suggest a divergence of the neural circuitry underlying the motor, innate behavior, and sleep phenotypes of AS mice from the circuitry responsible for seizure susceptibility and cortical EEG patterns.