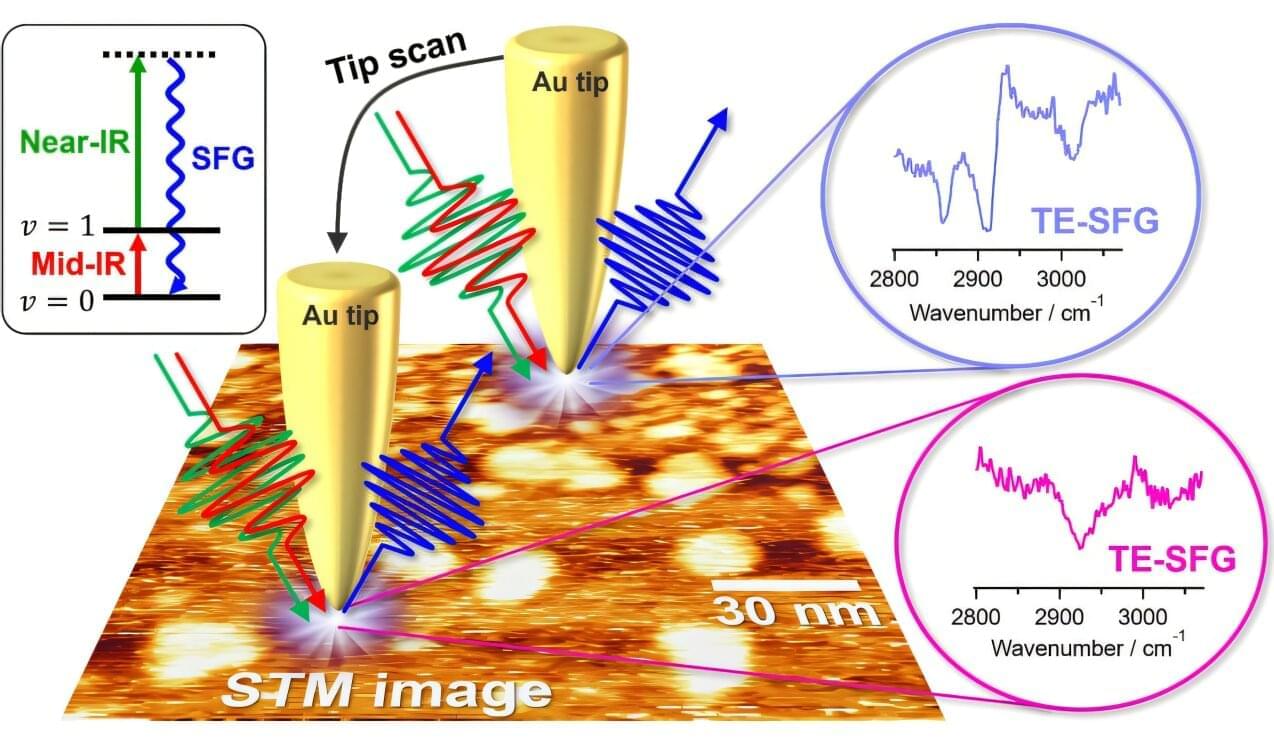
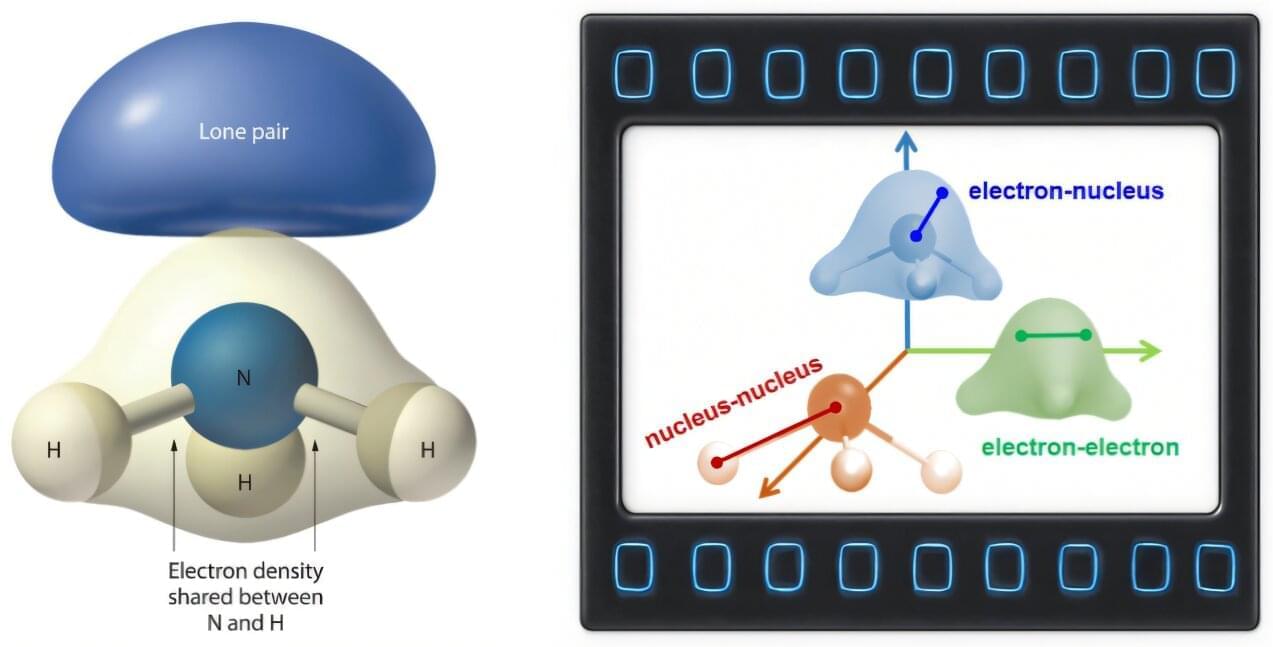
Sum-frequency generation (SFG) is a powerful vibrational spectroscopy that can selectively probe molecular structures at surfaces and interfaces, but its spatial resolution has been limited to the micrometer scale by the diffraction limit of light.
In a study published in The Journal of Physical Chemistry C, investigators overcame this limitation by utilizing a highly confined near field within a plasmonic nanogap and successfully extended the SFG spectroscopy into a nanoscopic regime with ~10-nm spatial resolution.
The team also established a comprehensive theoretical framework that accurately describes the microscopic mechanisms of this near-field SFG process. These experimental and theoretical achievements collectively represent a groundbreaking advancement in near-field second-order nonlinear nanospectroscopy, enabling direct access to correlated chemical and topographic information of interfacial molecular systems at the nanoscale.