It is still a challenge to elucidate kinetics and real-time transport routes for molecules through biological membranes in live cells. Currently, by developing and employing super-resolution microscopy; increasing evidence indicates channels and transporter nano-organization and dynamics within membranes play an important role in these regulatory mechanisms. Here we review recent advances and discuss the major advantages and disadvantages of using super-resolution microscopy to investigate protein organization and transport within plasma membranes.
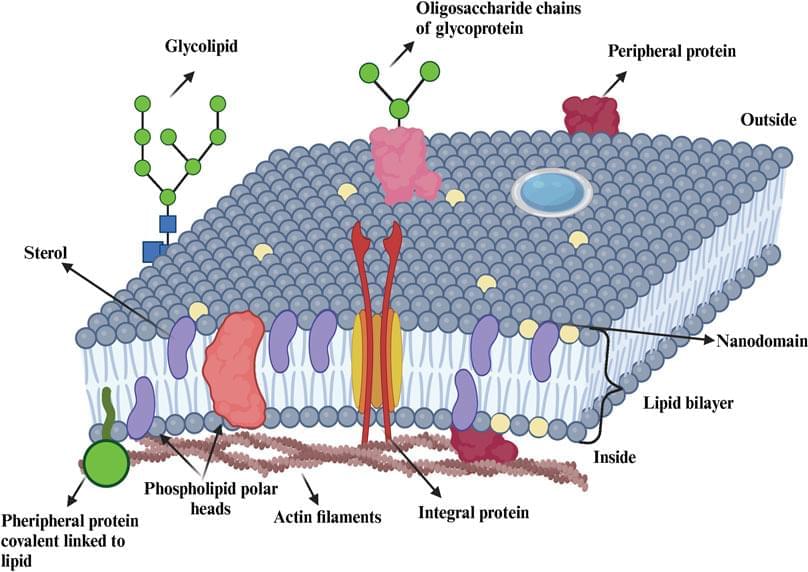
The mammalian plasma membrane (PM) is a complex assembly of lipids and proteins that separates the cell’s interior from the outside environment (Ingolfsson et al., 2014). The multiple collective processes that take place within membranes have a strong impact not only on the cellular behavior but also on its biochemistry. Understanding these processes poses a challenge due to the often complex and multiple interactions among membrane components (Stone et al., 2017). Moreover, the PM surface accommodates different types of lipid and protein clusters (Saka et al., 2014; Owen et al., 2012; Sezgin, 2017), even though the functional role of the clustering on the membrane surface has not yet been fully understood.