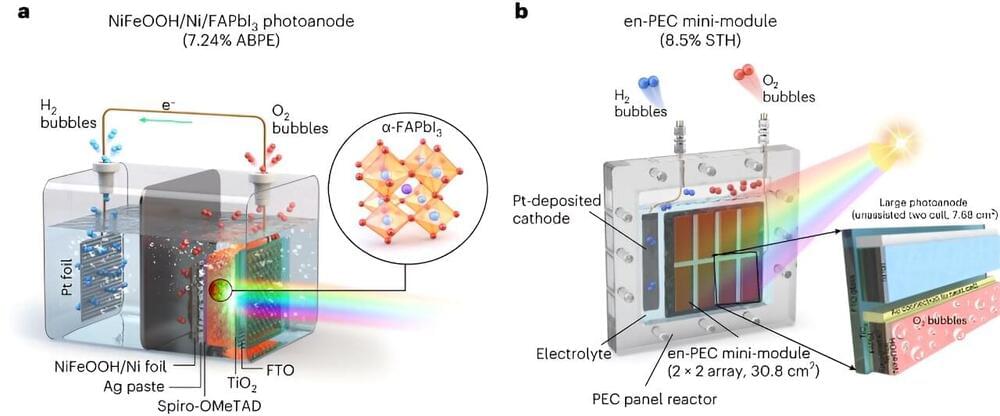
If realized using solar energy or other renewable energy, water splitting could be a promising way of sustainably producing hydrogen (H2) on a large-scale. Most photoelectrochemical water splitting systems proposed so far, however, have been found to be either inefficient, unstable, or difficult to implement on a large-scale.
Researchers at Ulsan National Institute of Science and Technology (UNIST) recently set out to develop a scalable and efficient photoelectrochemical (PEC) system to produce green hydrogen. Their proposed system, outlined in Nature Energy, is based on an innovative formamidinium lead triiodide (FAPbI3) perovskite-based photoanode, encapsulated by an Ni foil/NiFeOOH electrocatalyst.
“Our group has thoroughly studied the challenges associated with practical solar hydrogen production,” Jae Sung Lee, Professor of Energy & Chemical Engineering at UNIST and co-author of the paper, told Tech Xplore. “As summarized in our most recent review paper, minimum 10% of solar-to-hydrogen (STH) efficiency is required to develop viable practical PEC system, for which selecting an efficient material is the first criteria.”