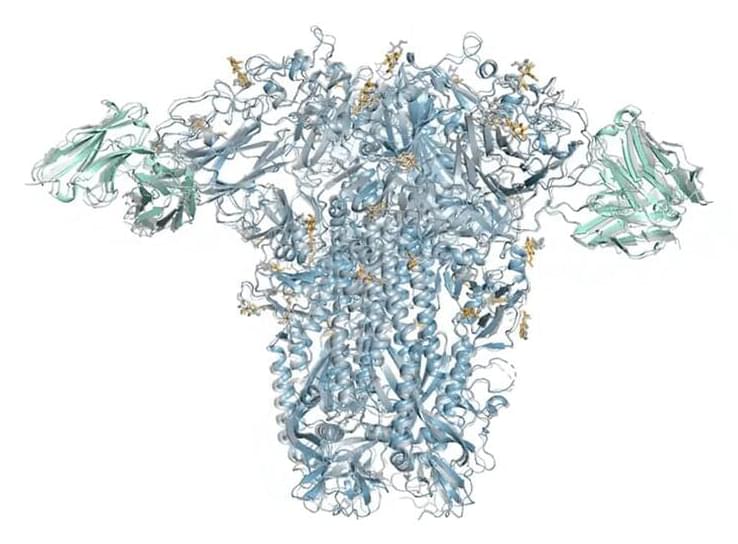
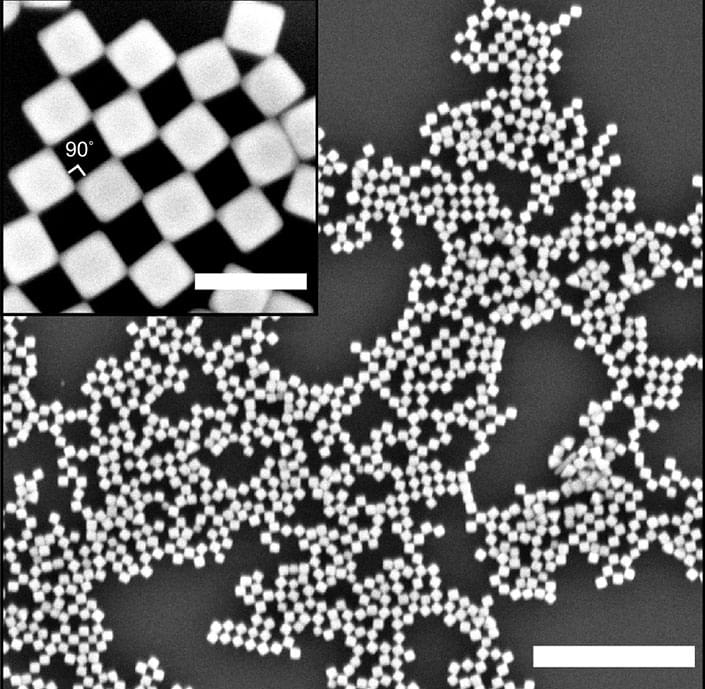
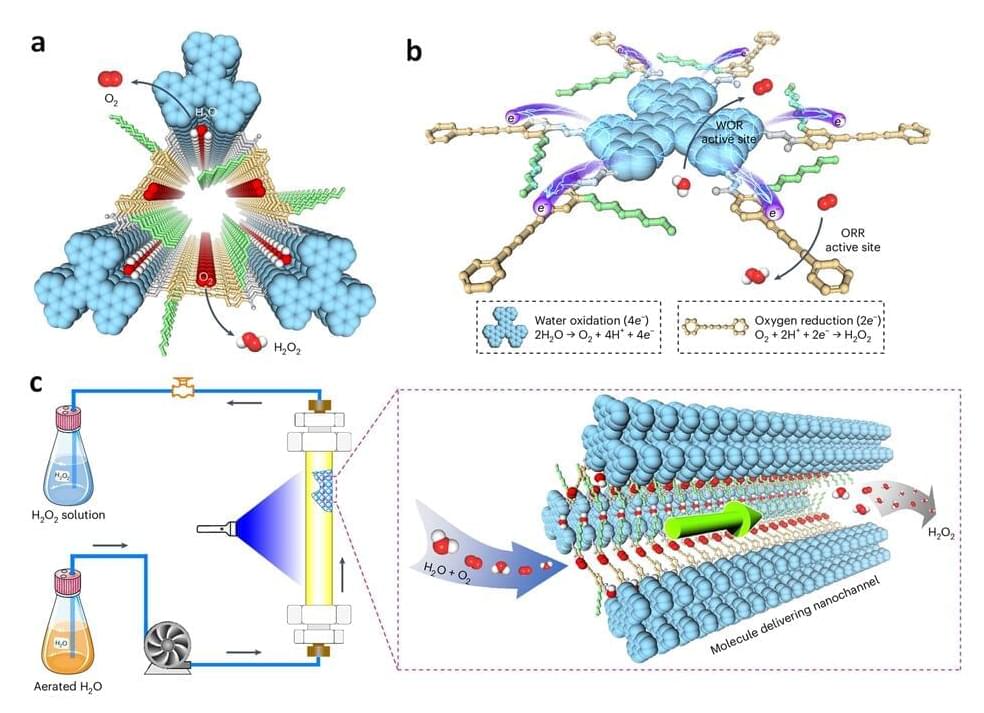
A series of advances in materials and design have enabled manufacturers to work at scales smaller than a billionth of a size to create devices and objects of nanoscopic dimensions. This is nanotechnology, which, although relatively new, produces materials and technologies already used in mass production.
The European Commission defines nano as any material that is at least 50% composed of particles between one and one hundred nanometers in size (i.e. one billionth of a meter, or one-millionth of a millimeter). Nanomaterials differ from conventional materials because of their unique properties such as higher electrical conductivity and mechanical strength, sensor technologies, and biomedical applications, and because they can create coatings that make surfaces more hydrophobic or self-cleaning.
The widespread use of nanotechnology is relatively new. Since 2000, nanomaterials have been used industrially as new research and experimental designs have made their effectiveness in different sectors clear. For example, in the health field, nanotechnology helps to reduce diagnostic errors and to develop nanobots (microscale robots) to repair and replace intercellular structures, or repair DNA molecules; in the chemical sector, it facilitates coating devices with nanoparticles to improve their smoothness and heat resistance; in manufacturing, materials developed with nanotechnology enhance the performance of the final product by improving heat resistance, strength, durability, and electrical conductivity.