Mice learn best when the opponent opposing forces of dopamine and serotonin work together, a new study shows, helping to resolve long-standing questions about the neuromodulators’ relationship.
In the intricate dance of learning and motivation, two key brain chemicals—dopamine (DA) and serotonin (5HT)—play opposing yet deeply interconnected roles. Scientists have long speculated how these neuromodulators work together to shape our ability to form new associations, but testing these theories directly has been a challenge.
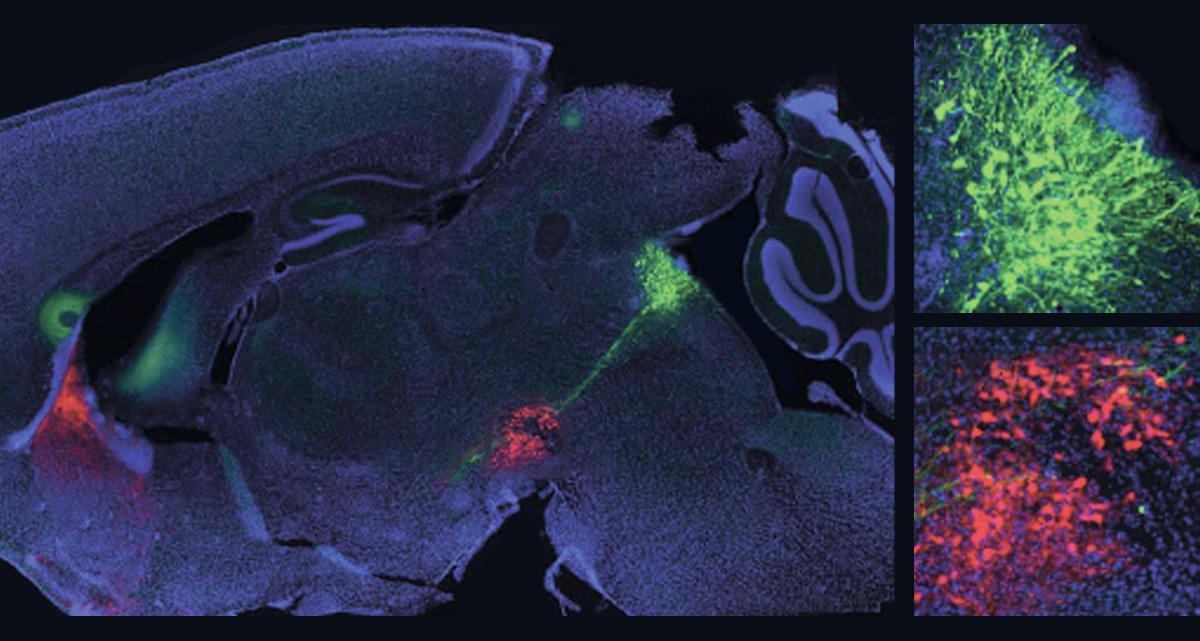
Now, researchers have developed a new mouse model that allows them to simultaneously study both dopamine and serotonin neurons in the brain. Their experiments focused on the nucleus accumbens (NAc), a region known for processing rewards. By monitoring neural activity, they found that receiving a reward boosts dopamine signals while simultaneously suppressing serotonin signals.
To understand how this dynamic affects learning, the team used optogenetics—a technique that uses light to control brain activity. They found that disrupting dopamine or serotonin alone caused only mild learning impairments. However, when both signals were suppressed together, the mice struggled significantly to learn from rewards. On the flip side, artificially recreating both dopamine and serotonin responses helped the mice learn more effectively than manipulating either signal alone.
These findings reveal that dopamine and serotonin work in opposition to control reinforcement and learning. Instead of acting in isolation, they create a delicate balance that shapes how we associate actions with rewards—providing new insights into how the brain learns and adapts.
Mice learn fastest and most reliably when they experience an increase in dopamine paired with an inhibition of serotonin, a new study shows.