Burkitt’s lymphoma is a rare and aggressive blood cancer characterized by a translocation of the MYC gene. It occurs most often in children and young adults. In recent years, CAR-T cell therapy—often referred to as a “living drug” and administered as a single dose—has been approved for certain types of blood cancer, offering hope for a cure even in severe cases. However, its effectiveness against Burkitt’s lymphoma has been limited. Moreover, developing drugs that directly target MYC—the root cause of this cancer—has proven challenging for decades.
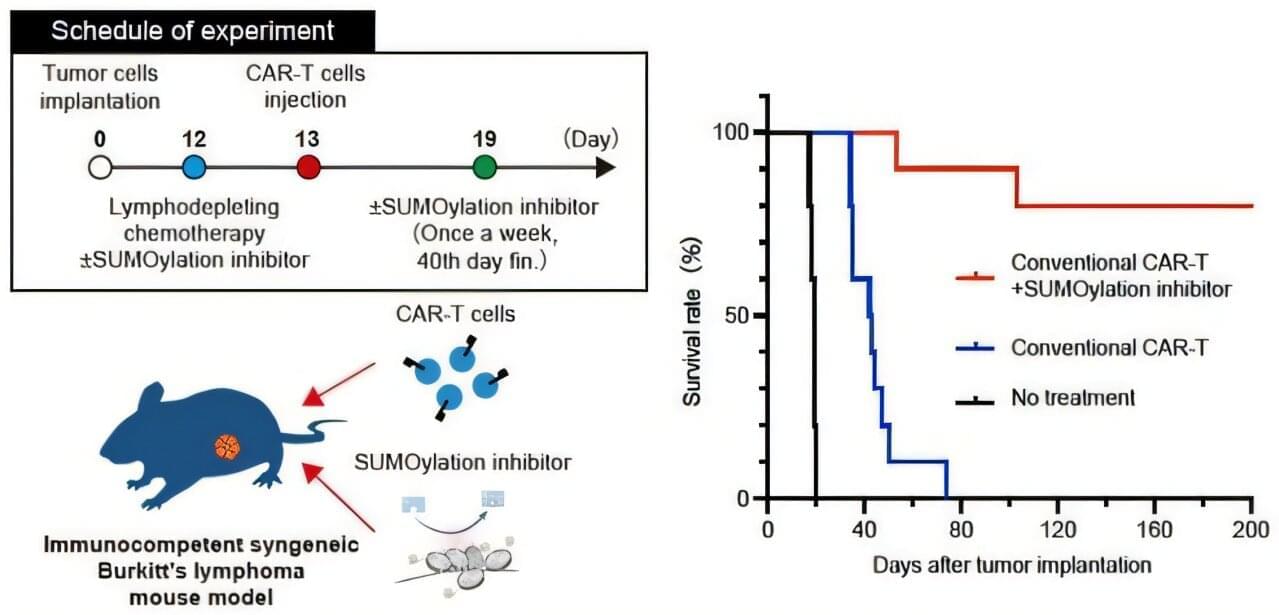
Recently, a study led by Dr. Hiroshi Kotani, Assistant Professor at Kanazawa University in collaboration with a scientist at Roswell Park Comprehensive Cancer Center in Buffalo, NY, U.S., revealed that a SUMOylation inhibitor can suppress MYC activity. Building on this finding, the research team investigated whether combining CAR-T therapy with the SUMOylation inhibitor TAK-981 could improve outcomes for Burkitt’s lymphoma. The research is published in Signal Transduction and Targeted Therapy.
The team first confirmed that the SUMOylation inhibitor effectively slowed the growth of Burkitt’s lymphoma cells and altered their signaling pathways. They then examined the inhibitor’s effect on CAR-T cells and discovered a dual role: While it initially activated the CAR-T cells in a way that could hinder long-term effectiveness, it also triggered a built-in “safety brake” mechanism. These insights suggested that using only a limited dose of the inhibitor could maximize the benefits of CAR-T therapy as a durable, living treatment.