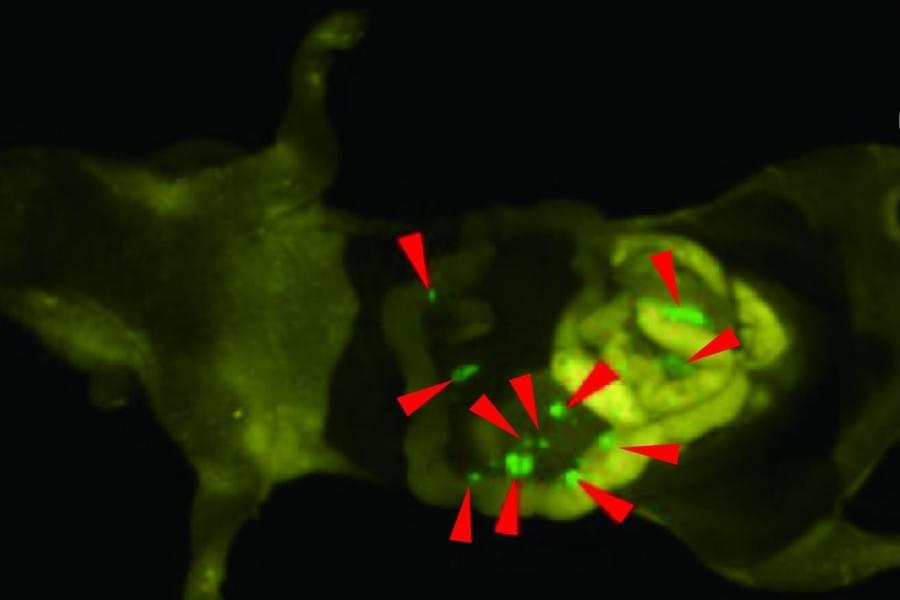
Successful cancer surgery depends on a surgeon’s ability to remove tumors, while minimizing harm to healthy tissues. Surgeons currently use glowing dyes which mark cancer cells to help differentiate from healthy cells, but these dyes aren’t perfect and will light up some healthy tissues too. For the first time, researchers including those from the University of Tokyo developed what they call a bioorthogonal fluorescence probe and a matching reporter enzyme that can activate the probe selectively at targeted tumor sites. This enables high-contrast tumor visualization with very low background. This study was performed in mice.
Cancer is a universal issue which affects uncountably many people around the world. Many will turn to surgery in the hope a surgeon will be able to completely remove a tumor leaving healthy tissues unaffected. Various tools and techniques have been developed over the years to improve the way these surgeries are performed, and visual imaging methods such as glowing dyes have proven to be very useful. But one drawback is that some probes can also be activated in healthy tissues by endogenous enzymes, creating background fluorescence and making it harder to judge what should be removed. The opposite is also possible, where cancer cells are left unmarked and are missed during surgery, increasing the chance of recurrence.
“Our group acknowledged this current shortcoming and improved upon this way to make cancer cells light up inside the body. In tests on mice, we delivered a special enzyme to tumors and used a fluorescence probe that only turns on when that enzyme is present,” said Associate Professor Ryosuke Kojima from the Laboratory of Chemical Biology and Molecular Imaging at the University of Tokyo. “Older probes often light up healthy tissue by mistake, creating background noise, but our highly selective, or bioorthogonal, dye probe is designed to stay completely off unless it meets its matching engineered enzyme. We essentially trained the enzyme through repeated mutation and selection, a form of directed evolution, so it could activate the probe strongly enough to work inside living animals.”