Breakthroughs in computing are supercharging a field of science dedicated to building synthetic organisms from scratch.



Among adults with Type2Diabetes and liver cirrhosis, SGLT2 inhibitor use was associated with lower risks of end-stage kidney disease, cardiovascular events, mortality, and hepatic decompensation compared with DPP4 inhibitors.
Importance Type 2 diabetes (T2D) and liver cirrhosis frequently coexist, creating a high-risk population for adverse outcomes. Patients with both conditions face elevated risks of kidney and cardiovascular complications, yet evidence regarding optimal antidiabetic therapy in this vulnerable population remains limited.
Objective To evaluate the association of sodium-glucose cotransporter–2 inhibitor (SGLT2i) vs dipeptidyl peptidase–4 inhibitor (DPP4i) use with kidney outcomes, cardiovascular events, and hepatic decompensation in patients with concurrent T2D and liver cirrhosis.
Design, Setting, and Participants This nationwide retrospective cohort study utilized data from the Taiwan’s National Health Insurance Database between May 2016 and December 2023. Adults with both T2D and liver cirrhosis who initiated either SGLT2is or DPP4is were included.

SINCE the 1980s, researchers have worked tirelessly to develop effective treatments that can suppress the HIV virus to undetectable levels. As a result, by taking a single daily dose of an antiretroviral (ARV) medicine, people with HIV can now expect to live long and healthy lives – as well as prevent transmission to others.
The impact has been dramatic, with annual global AIDS-related deaths having fallen from 2.1 million at the peak in 2004 to 630,000 in 2024. The number of new acquisitions has plummeted too: an estimated 1.3 million people acquired the virus in 2024, marking a 60 per cent decline since the peak in 1995.
“We’re at a pivotal moment in the quest to end the HIV epidemic,” says Jean van Wyk, chief medical officer at ViiV Healthcare. This quest is to meet the UN’s 2030 goals that ViiV has been involved with since the 1980s as the pioneers of the first ARV medicine.

Shingles, a viral rash, can be incredibly painful. Vaccination can help prevent the infection, but new research is showing the shingles vaccine may also have another benefit: protection against the development of dementia. With more than 40 percent of Americans estimated to develop dementia at some point in their lives, this discovery could have groundbreaking implications for our health. But what explains the link between the shingles vaccine and reduced dementia risk?
Recent research is part of a growing body of evidence that vaccination against shingles—and potentially other infections—can help prevent and delay the progression of dementia.
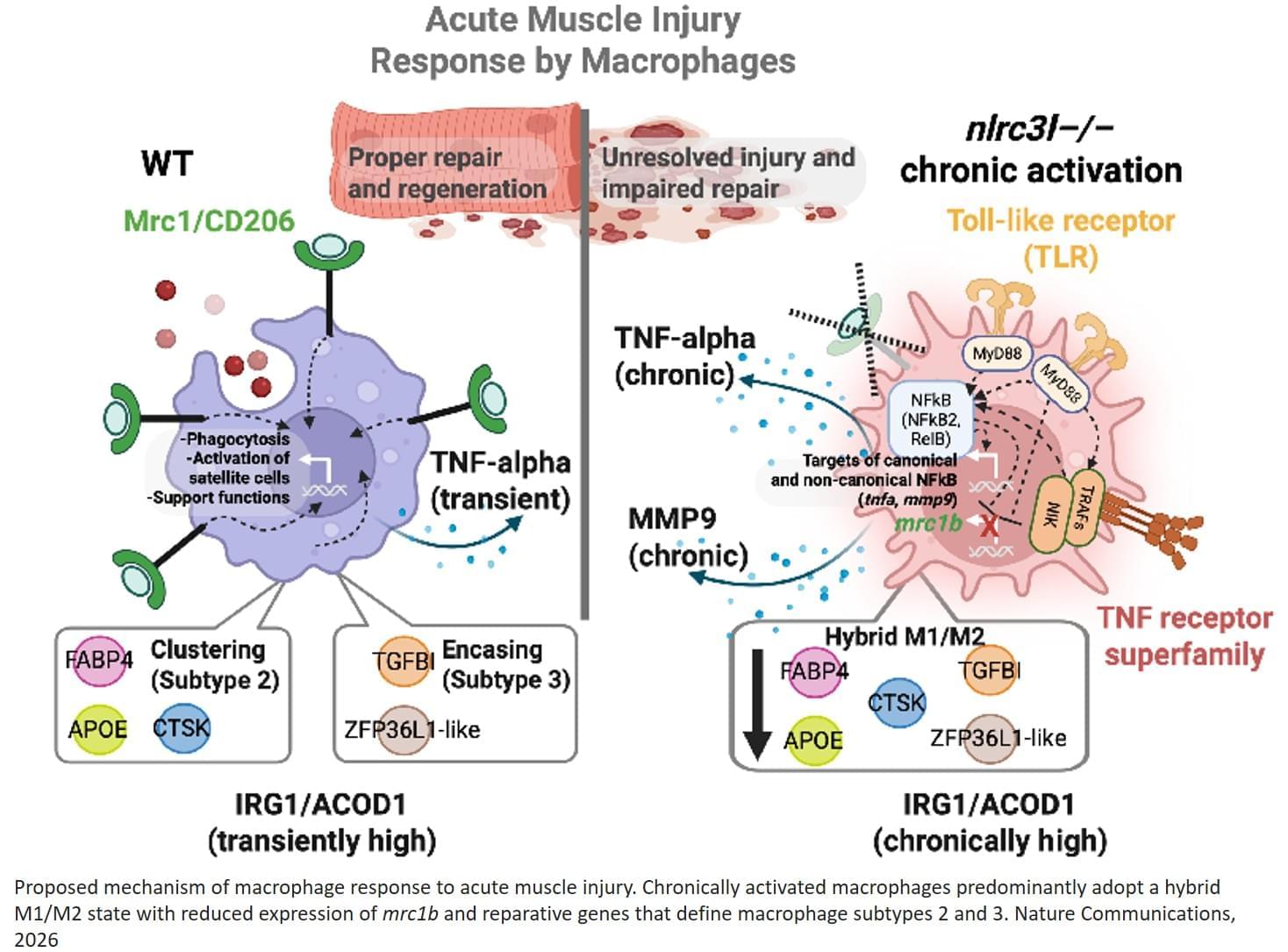
TIL therapy for glioblastoma.
Tumor infiltrating lymphocyte (TIL) therapy has demonstrated encouraging efficacy in melanoma and nonsmall-cell lung cancer (NSCLC), and is now being explored for glioblastoma despite its immunologically ‘cold’ microenvironment.
Recent studies confirm that functional TILs can be expanded from cold tumors such as glioblastoma, including solid tumor resections and aspirates, overcoming previous feasibility concerns.
Advances in cytokine support, gene editing, and artificial antigen-presenting cells (APCs) are improving TIL persistence, cytotoxicity, and manufacturing scalability.
Focused ultrasound and nanoparticle delivery offer innovative solutions to enhance TIL infiltration across the blood– brain barrier. Integration of spatial multi-omics enables high-resolution mapping of immune niches and identification of tumorreactive clones.
Combination strategies with checkpoint blockade, myeloid modulation, and oncolytic virotherapy are emerging as rational paths to enhance TIL efficacy sciencenewshighlights ScienceMission https://sciencemission.com/TIL-therapy-17895

Unless you’ve been extremely lucky, you’ve likely been wounded, be it a knife cut while cooking or a sports injury. To remedy this unpleasant experience, you’ve taken some version of the following steps: clean the wound, disinfect the area, and apply a plaster or bandage. While a common and simple first-aid skill, this wound healing process has existed since ancient times.
Furthermore, there are wound cases, especially chronic wounds that arise from conditions such as diabetes, that can be more severe than one might expect. The 5-year survival rate of patients with chronic wounds is about 70%, which is worse than that of breast cancer, prostate cancer and other diseases. In addition, treating wounds adds to the cost of care, leading to about $28 billion per year in the U.S. alone.
Following the traditional use case, the main function of bandages for acute or chronic wound care has been to protect the injured area from external factors that could worsen the injury, such as dirt, bacterial infection and friction. Over the centuries since the inception of wound dressing, some changes have taken place. These have mostly related to the material of bandages, such as stronger-adhering waterproof ones; but the role of the bandage has retained its passive role.


The eyes—specifically, the outer area of the retina—may provide a window into early detection of Alzheimer’s disease (AD) long before irreversible brain damage has occurred, according to new research from Houston Methodist. This discovery could dramatically change how the disease is diagnosed, monitored and treated.
“Retinal Müller glia alterations and their impact on ocular glymphatic clearance in an Alzheimer’s disease mouse model,” is online and will appear in an upcoming edition of the Journal of Alzheimer’s Disease. Led by Stephen Wong, Ph.D., the John S. Dunn Presidential Distinguished Chair in Biomedical Engineering at Houston Methodist and director of T. T. & W. F. Chao Center for BRAIN, the study reveals how the peripheral retina (versus the central retina) could be a window into early diagnosis of AD.
“The eyes are indeed a window into the brain, but our study reveals that we have been looking at the wrong part of the window,” Wong said. “While most clinical eye exams focus on the central retina, the most critical early indicators of AD appear to be hidden at the periphery of the eye. By identifying these retinal changes that occur before the brain’s ‘plumbing’ system fails, doctors may eventually be able to use routine eye exams to catch and treat the disease years before memory loss begins.”
