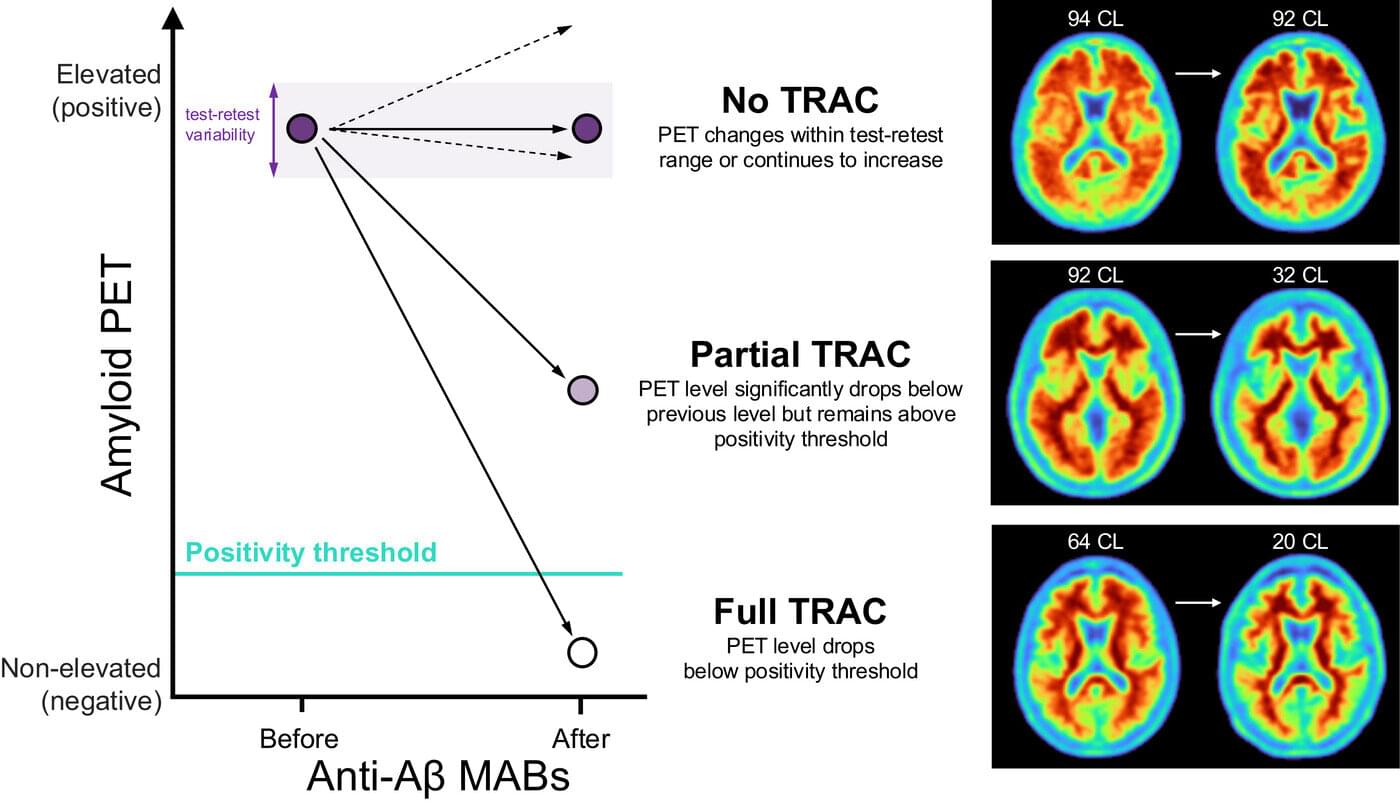
A large-scale screen of tuberculosis proteins has revealed several possible antigens that could be developed as a new vaccine for TB, the world’s deadliest infectious disease.
In the new study, a team of MIT biological engineers was able to identify a handful of immunogenic peptides, out of more than 4,000 bacterial proteins, that appear to stimulate a strong response from a type of T cells responsible for orchestrating immune cells’ response to infection.
There is currently only one vaccine for tuberculosis, known as BCG, which is a weakened version of a bacterium that causes TB in cows. This vaccine is widely administered in some parts of the world, but it poorly protects adults against pulmonary TB. Worldwide, tuberculosis kills more than 1 million people every year.