The procedure works by removing the vitreous gel that sits between the eye’s lens and retina, and replacing it with saline solution, researchers from the University of Washington, found.
Category: biotech/medical – Page 2603
A ‘living bandage’ made from stem cells, which could revolutionise the treatment and prognosis of a common sporting knee injury, has been trialled in humans for the first time by scientists at the Universities of Liverpool and Bristol.
Meniscal tears are suffered by over one million people a year in the US and Europe alone and are particularly common in contact sports like football and rugby. 90% or more of tears occur in the white zone of meniscus which lacks a blood supply, making them difficult to repair. Many professional sports players opt to have the torn tissue removed altogether, risking osteoarthritis in later life.
The Cell Bandage has been developed by spin-out company Azellon, and is designed to enable the meniscal tear to repair itself by encouraging cell growth in the affected tissue.
Check out the LEAF interview with Synthetic Biology company CellAge who plan to use their technology to create aging biomarkers for the research community to use for free as well as new approaches to removing senescent cells.
CellAge are using synthetic biology to remove senescent cells that accumulate with age and contribute to disease. We took the time to interview them about their technology, treating age-related diseases and their plans for the future.
You can also check out their campaign on Lifespan.io:
https://www.lifespan.io/campaigns/cellage-targeting-senescen…c-biology/
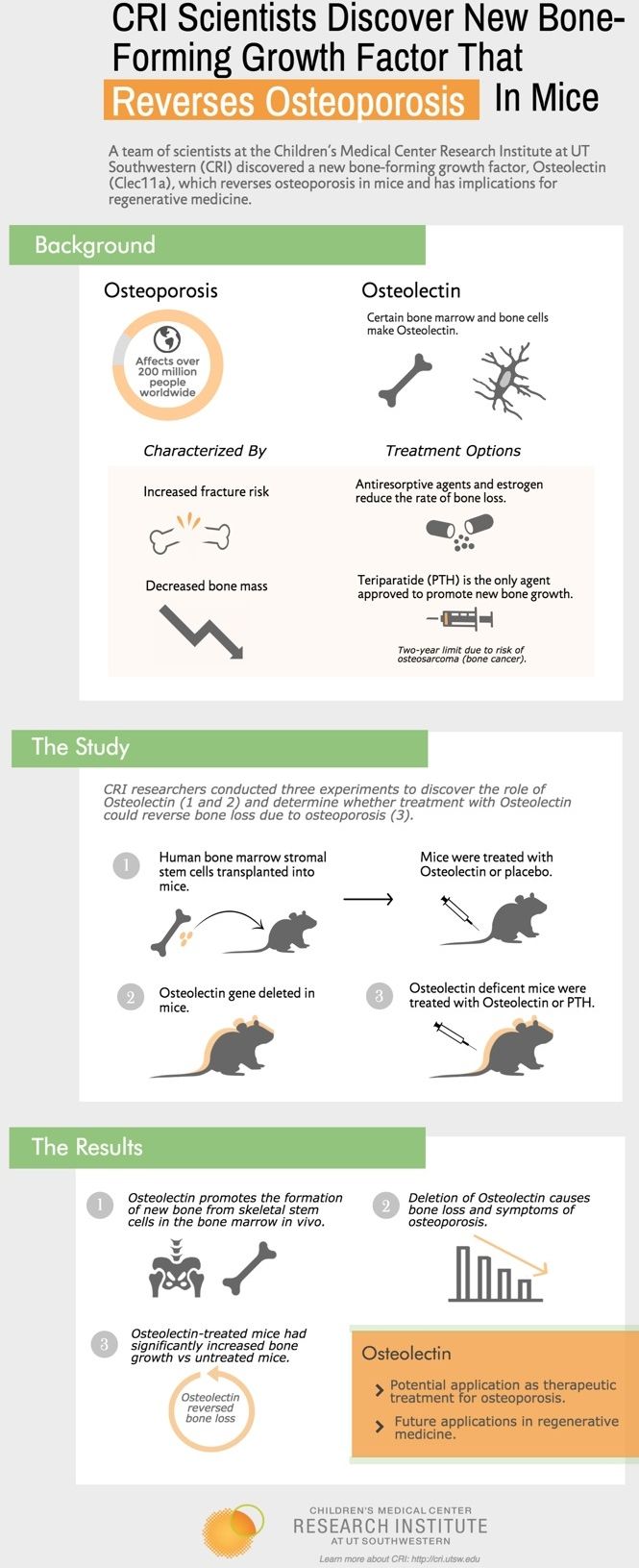
Progress with treating osteoporosis.
A team of scientists at the Children’s Medical Center Research Institute at UT Southwestern (CRI) discovered a new bone-forming growth factor, Osteolectin (Clec11a), which reverses osteoporosis in mice and has implications for regenerative medicine.
Although Osteolectin is known to be made by certain bone marrow and bone cells, CRI researchers are the first to show Osteolectin promotes the formation of new bone from skeletal stem cells in the bone marrow. The study, published in eLife, also found that deletion of Osteolectin in mice causes accelerated bone loss during adulthood and symptoms of osteoporosis, such as reduced bone strength and delayed fracture healing.
“These results demonstrate the important role Osteolectin plays in new bone formation and maintaining adult bone mass. This study opens up the possibility of using this growth factor to treat diseases like osteoporosis,” said Dr. Sean Morrison, who led the team that made the discovery. Dr. Morrison, CRI Director, holds the Mary McDermott Cook Chair in Pediatric Genetics at UT Southwestern Medical Center, and the Kathryne and Gene Bishop Distinguished Chair in Pediatric Research at Children’s Research Institute at UT Southwestern.
Dr. Aubrey de Grey on the case again in this amusing video.
Dr. Aubrey de Grey in a new video where people ask questions via Twitter. It is a bit tongue in cheek and sorry about the title but hopefully you will enjoy it,
If you liked this video and agree that eliminating age-related diseases is a good idea please consider visiting our website and making a donation for science on the link below:
An interesting but predictably hyped research study currently doing the rounds. Epigentic changes are one of the Hallmarks of Aging and this study reinforces their importance despite the usual media hype.
Graying hair, crow’s feet, an injury that’s taking longer to heal than when we were 20—faced with the unmistakable signs of aging, most of us have had a least one fantasy of turning back time. Now, scientists at the Salk Institute have found that intermittent expression of genes normally associated with an embryonic state can reverse the hallmarks of old age.
This approach, which not only prompted human skin cells in a dish to look and behave young again, also resulted in the rejuvenation of mice with a premature aging disease, countering signs of aging and increasing the animals’ lifespan by 30 percent. The early-stage work provides insight both into the cellular drivers of aging and possible therapeutic approaches for improving human health and longevity.
“Our study shows that aging may not have to proceed in one single direction,” says Juan Carlos Izpisua Belmonte, a professor in Salk’s Gene Expression Laboratory and senior author of the paper appearing in the December 15, 2016 issue of Cell. “It has plasticity and, with careful modulation, aging might be reversed.”
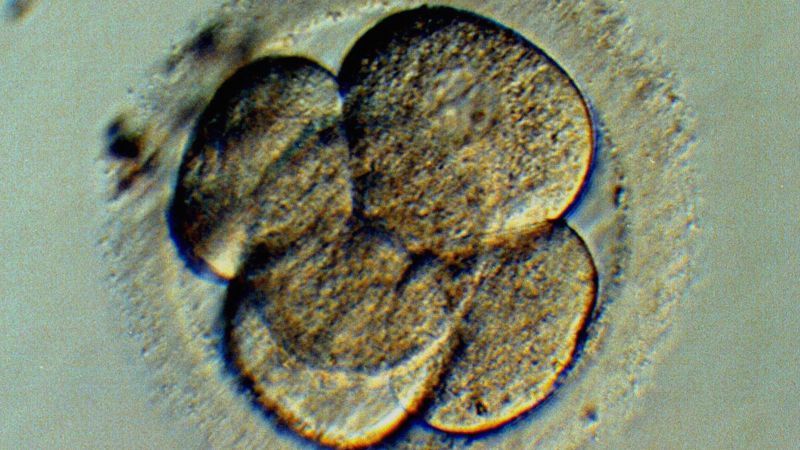
In September, a doctor named John Zhang announced that a baby, created via a complicated fertility treatment involving DNA contributions from three people, was successfully delivered the previous April. Now the U.K. has opened the way for more attempts at creating babies with three parents.
The fertility treatment involves sperm, an egg from the prospective mother, and an egg from a donor and has been used to help women who have mitochondrial issues with their eggs, replacing the nucleus DNA of those eggs with that of donor, either before or after fertilization. The embryo then carries the donor’s mitochondrial DNA, which amounts to less than 1% of the resulting child’s genes. CBS News reports that on Thursday, Britain’s fertility regulator, Human Fertilisation and Embryology Authority, approved the technique.
We already know that excessive amounts of stress long term can cause certain individuals with certain predisposition cancer genetic mutations can cause cancer such as breast cancer. So, not surprise to see this.
In some situations, people who got hurt, replay the disturbing moment in their heads for many times and for many days. Every repetition you make usually causes more intense feelings making the situation worse.
Thanks to modern medicine, there is now proof that keeping these emotions inside you can have negative effects on your overall health. That’s why we would like to discuss forgiveness.
What combinations of mutations help cancer cells survive? Which cells in the brain are involved in the onset of Alzheimer’s? How do immune cells conduct their convoluted decision-making processes? Researchers at the Weizmann Institute of Science have now combined two powerful research tools — CRISPR gene editing and single cell genomic profiling — in a method that may finally help us get answers to these questions and many more.
The new technology enables researchers to manipulate gene functions within single cells, and understand the results of each change in extremely high resolution. A single experiment with this method, say the scientists, may be equal to thousands of experiments conducted using previous approaches, and it may advance the field of genetic engineering for medical applications.
The gene-editing technique CRISPR is already transforming biology research around the world, and its clinical use in humans is just around the corner. CRISPR was first discovered in bacteria as a primitive acquired immune system, which cuts and pastes viral DNA into their own genomes to fight viruses. In recent years, this bacterial system has been adopted by researchers to snip out or insert nearly any gene in any organism or cell, quickly and efficiently. “But CRISPR, on its own, is a blunt research tool, since we often have trouble observing or understanding the outcome of this genomic editing,” says Prof. Ido Amit of the Weizmann Institute of Science’s Immunology Department, who led the study. “Most studies so far have looked for black-or-white types of effects,” adds Dr. Diego Jaitin, of Amit’s lab group, “but the majority of processes in the body are complex and even chaotic.”