Interesting read on recent Gastric Cancer research. I do a lot of work with the National Esophageal Cancer (EC) Awareness Association; I can tell you that this disease is truly a killer as gastric related cancers are horrible to detect early enough and have a horrible record of reoccurring. Survival rates are some of the worst and today the rates of EC have skyrocketed especially in the younger age groups such as 25 to 35 year olds.
When you work for these foundations, read the stories from patients and their families looking for answers and help with everything from help on what types of food can their love eat and hopefully keep down for nutrition, to how can they get help with transportation to simply go to work or the doctor as meds restrictions on driving, to knowing the end is near and how to prepare, etc. The worst ones are the 27 to 36 yr old fathers and mothers whose love one is saying good bye to the person they married only recently married the year before or spent 7 years with. This is why I work for my foundations as every small step does in the end create a larger impact in the end and hopefully helps us finally beat this disease.
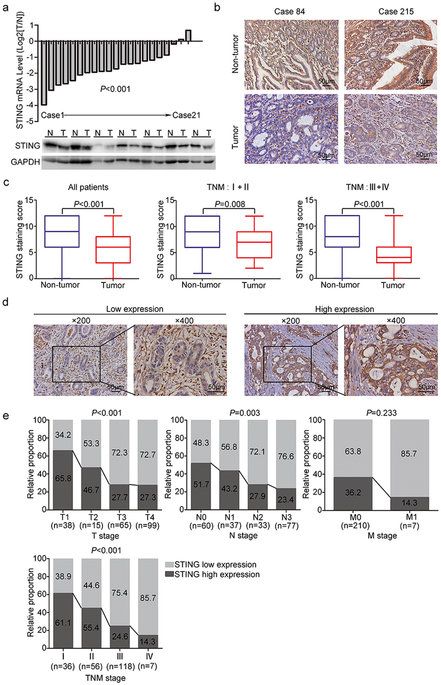
STING (stimulator of interferon genes) has recently been found to play an important role in host defenses against virus and intracellular bacteria via the regulation of type-I IFN signaling and innate immunity. Chronic infection with Helicobacter pylori is identified as the strongest risk factor for gastric cancer. Thus, we aim to explore the function of STING signaling in the development of gastric cancer. Immunohistochemistry was used to detect STING expression in 217 gastric cancer patients who underwent surgical resection. STING protein expression was remarkably decreased in tumor tissues compared to non-tumor tissues, and low STING staining intensity was positively correlated with tumor size, tumor invasion depth, lymph mode metastasis, TNM stage, and reduced patients’ survival. Multivariate analysis identified STING as an independent prognostic factor, which could improve the predictive accuracy for overall survival when incorporated into TNM staging system. In vitro studies revealed that knock-down of STING promoted colony formation, viability, migration and invasion of gastric cancer cells, and also led to a defect in cytosolic DNA sensing. Besides, chronic H. pylori infection up-regulated STING expression and activated STING signaling in mice. In conclusion, STING was proposed as a novel independent prognostic factor and potential immunotherapeutic target for gastric cancer.